Label: POTASSIUM CHLORIDE FOR ORAL SOLUTION powder, for solution
- NDC Code(s): 82983-403-01, 82983-403-10, 82983-403-30
- Packager: Ajenat Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTASSIUM CHLORIDE FOR ORAL SOLUTION safely and effectively. See full prescribing information for POTASSIUM CHLORIDE FOR ORAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPotassium Chloride is indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis, in patients for whom dietary management with potassium-rich foods or diuretic ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration and Monitoring - If serum potassium concentration is <2.5 mEq/L, use intravenous potassium instead of oral supplementation. Monitoring - Monitor serum potassium and adjust ...
-
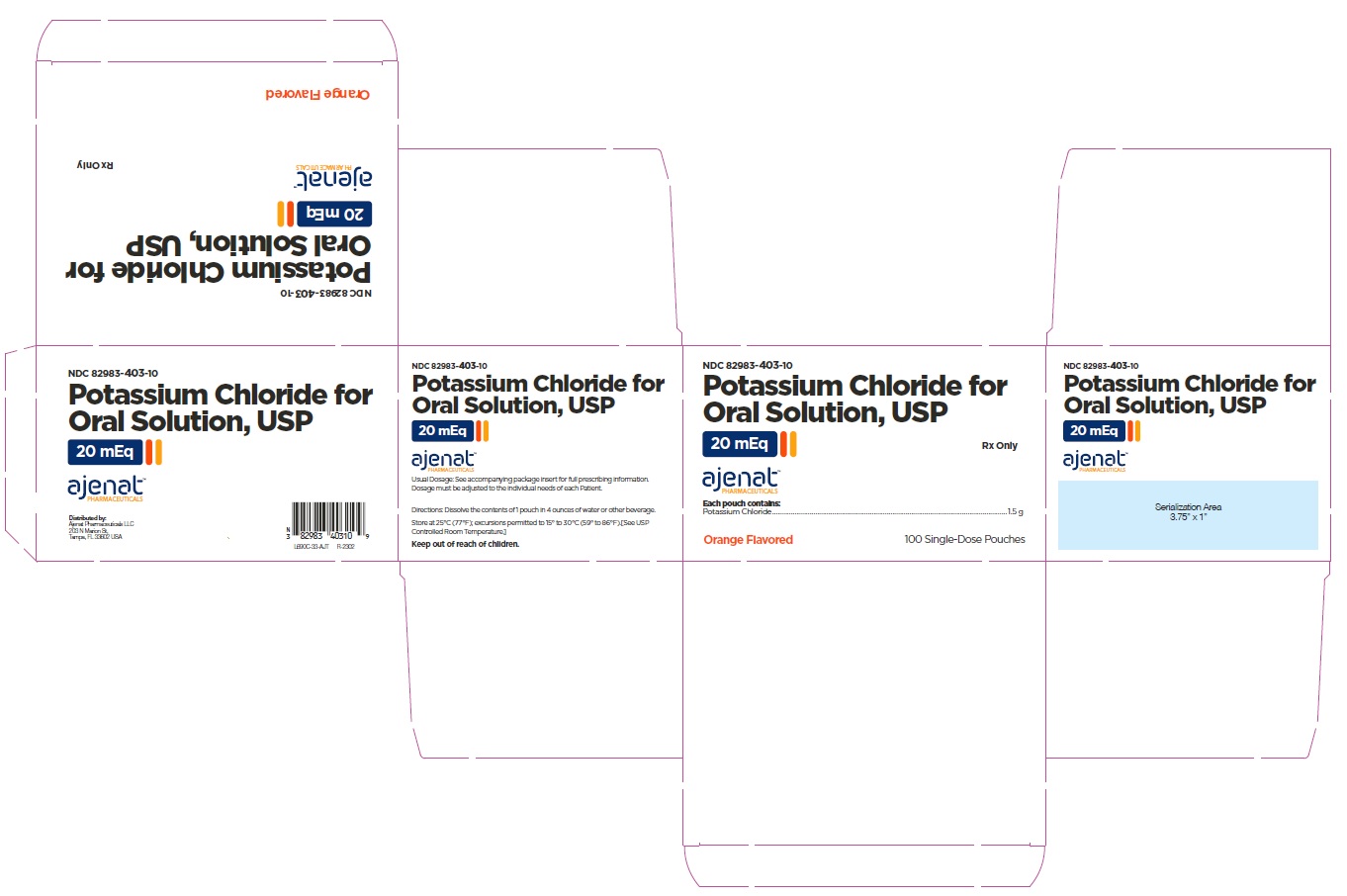
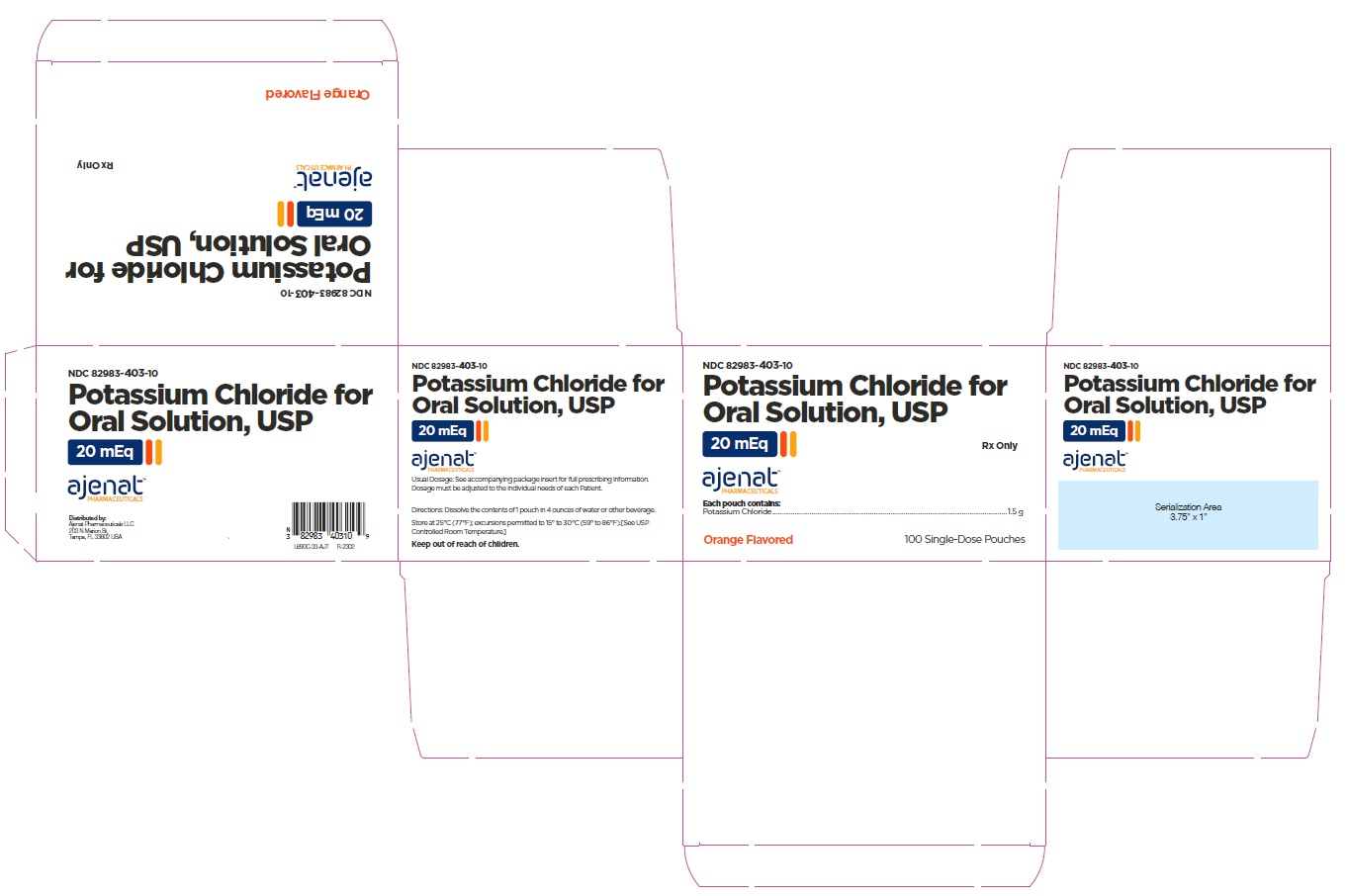
3 DOSAGE FORMS AND STRENGTHSEach pouch contains 1.5 g of potassium chloride supplying 20 mEq of potassium and 20 mEq of chloride.
-
4 CONTRAINDICATIONSPotassium chloride is contraindicated in patients on potassium sparing diuretics.
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Irritation - May cause gastrointestinal irritation. Increased dilution of the solution and taking with meals may reduce gastrointestinal irritation [ see Dosage and ...
-
6 ADVERSE REACTIONSThe most common adverse reactions to oral potassium salts are nausea, vomiting, flatulence, abdominal pain/discomfort, and diarrhea.
-
7 DRUG INTERACTIONS7.1 Potassium-Sparing Diuretics - Use with potassium-sparing diuretic can produce severe hyperkalemia. Avoid concomitant use. 7.2 Angiotensin-Converting Enzyme Inhibitors - Use with angiotensin ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - Animal reproduction studies have not been conducted with potassium chloride. It is unlikely that potassium supplementation that does not lead to hyperkalemia ...
-
10 OVERDOSAGE10.1 Symptoms - The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are ...
-
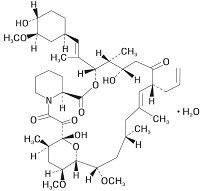
11 DESCRIPTIONPotassium Chloride is a white crystalline or colorless solid. It is soluble in water and slightly soluble in alcohol. Chemically, Potassium Chloride is K-Cl with a molecular mass of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The potassium ion (K+) is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes including ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPotassium Chloride for Oral Solution, is a light pink to orange powder available in one strength as follows: 20 mEq - NDC# 82983-403-01 pouch. Each pouch contains 1.5 g of potassium chloride ...
-
PRINCIPAL DISPLAY PANELNDC# 82983-403-30 - Potassium Chloride for Oral Solution, USP - 20 mEq - Rx only - 30 Single-Dose Pouches - NDC# 82983-403-10 - Potassium Chloride for Oral Solution, USP - 20 mEq - Rx only - 100 Single-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information