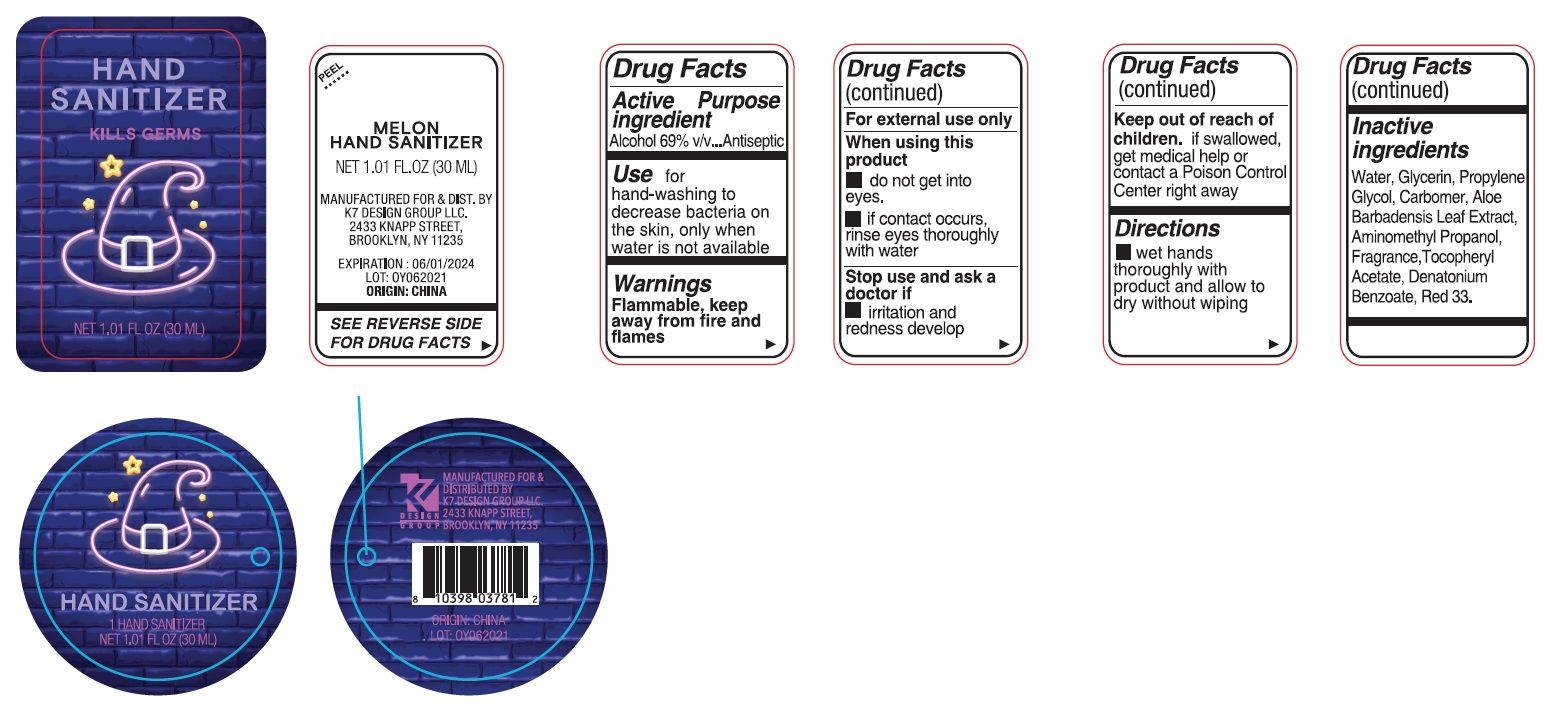
Label: MELON HAND SANITIZER- alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 74177-994-01 - Packager: K7 Design Group Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active ingredientAlcohol 69% v/v
-
PurposeAntiseptic
-
Usefor hand-washing to decrease bacteria on the skin, only when water is not available
-
WarningsFlammable, keep away from fire and flames - For external use only - When using this product - do not get into eyes. if contact occurs, rinse eyes thoroughly with water - Stop use and ask a ...
-
Directionswet hands thoroughly with product and allow to dry without wiping
-
Inactive ingredientsWater, Glycerin, Propylene Glycol, Carbomer, Aloe Barbadensis Leaf Extract, Aminomethyl Propanol, Fragrance, Tocopheryl Acetate, Denatonium Benzoate, Red 33.
-
Company InformationMANUFACTURED FOR & DIST. BY K7 DESIGN GROUP LLC - 2344 KNAPP STREET - BROOKLYN, NY 11235
-
Product Packaging
-
INGREDIENTS AND APPEARANCEProduct Information

