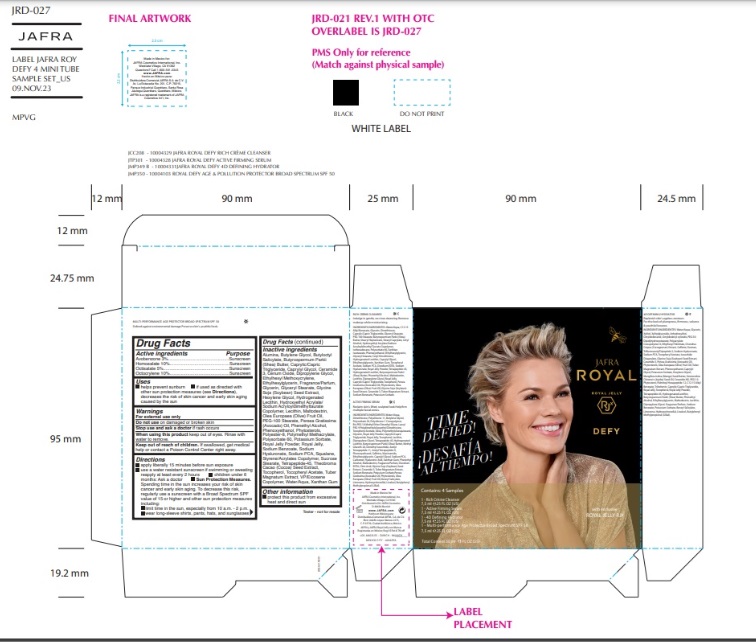
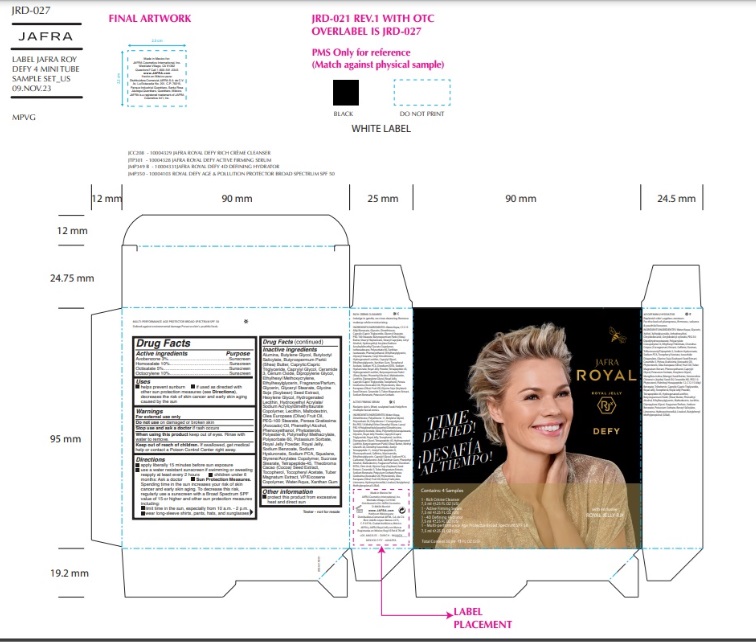
Label: JAFRA ROYAL DEFY SAMPLE KIT- avobenzone, homosalate, octisalate, octocrylene kit
ROYAL DEFY MULTI-PERFORMANCE AGE PROTECTOR BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene lotion
JAFRA ROYAL DEFY TRAVEL KIT- avobenzone, homosalate, octisalate, octocrylene kit
-
NDC Code(s):
68828-760-01,
68828-761-01,
68828-762-01,
68828-763-01, view more68828-764-01
- Packager: Jafra cosmetics International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every 2 hours.
- use a water-resistant sunscreen if swimming or sweating
- children under 6 months : Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
Water, Butyloctyl Salicylate, VP/Eicosene Copolymer, Polyester-8, Glyceryl Stearate,PEG-100 Stearate, Styrene/Acrylates Copolymer, Ethylhexyl Methoxycrylene,Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Sucrose Stearate,Squalane, Phenoxyethanol, Caprylyl Glycol, Xanthan Gum, Polymethyl Methacrylate,Polysorbate 60, Potassium Sorbate, Royal Jelly Powder, Tocopheryl Acetate, Hexylene Glycol, Persea Gratissima (Avocado) Oil, Sodium PCA, Caprylic/Capric Triglyceride,Phytosterols, Cerium Oxide, Glycerin, Olea Europaea (Olive) Fruit Oil, Glycine Soja(Soybean) Seed Extract, Butyrospermum Parkii (Shea) Butter, Royal Jelly, Dipropylene Glycol, Lecithin, Alumina, Sodium Hyaluronate, Hydrogenated Lecithin, Butylene Glycol, Tocopherol, Phenethyl Alcohol, Tuber Magnatum Extract, Ceramide NP, Ethylhexylglycerin, Maltodextrin, Theobroma Cacao (Cocoa) Seed Extract, Tetrapeptide-45, Sodium Benzoate, Farnesol, Fragrance/Parfum, Benzyl Salicylate, Butylphenyl Methylpropional, Linalool, Hydroxycitronellal, Limonene
- Questions?
- Product label
-
INGREDIENTS AND APPEARANCE
JAFRA ROYAL DEFY SAMPLE KIT
avobenzone, homosalate, octisalate, octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-760 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-760-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 01/13/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 7.5 mL Part 2 1 TUBE 7.5 mL Part 3 1 TUBE 7.5 mL Part 4 1 TUBE 7.5 mL Part 1 of 4 ACTIVE FIRMING SERUM
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) INGR HYALURONIC ACID (UNII: S270N0TRQY) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR WATER (UNII: 059QF0KO0R) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR BIS-PEG-18 METHYL ETHER DIMETHYL SILANE (UNII: OEB4R3WW9C) INGR LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR SILICON DIOXIDE (UNII: ETJ7Z6XBU4) INGR POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ROYAL JELLY (UNII: L497I37F0C) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR METHYL GLUCETH-20 (UNII: J3QD0LD11P) INGR DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) INGR ACETYL TETRAPEPTIDE-11 (UNII: 0R3VC3BV8I) INGR ACETYL TETRAPEPTIDE-9 (UNII: VMO8OOD3V0) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CAFFEINE (UNII: 3G6A5W338E) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR FARNESOL (UNII: EB41QIU6JL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 2 of 4 4D DEFINING HYDRATOR
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR XYLITOL (UNII: VCQ006KQ1E) INGR XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) INGR ANHYDROXYLITOL (UNII: 8XWR7NN42F) INGR OCTYLDODECANOL (UNII: 461N1O614Y) INGR OCTYLDODECYL XYLOSIDE (UNII: 8Z6VNR46QM) INGR PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) INGR AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) INGR ETHYLHEXYL PALMITATE (UNII: 2865993309) INGR CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) INGR CAFFEINE (UNII: 3G6A5W338E) INGR DEXTRAN 40 (UNII: K3R6ZDH4DU) INGR TRIFLUOROACETYL TRIPEPTIDE-2 (UNII: E6WT9V3SGO) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR ISOSORBIDE DICAPRYLATE (UNII: 0IK29C4889) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) INGR JOJOBA OIL (UNII: 724GKU717M) INGR CERAMIDE NG (UNII: C04977SRJ5) INGR PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR TRIBEHENIN (UNII: 8OC9U7TQZ0) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR ROYAL JELLY (UNII: L497I37F0C) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR FARNESOL (UNII: EB41QIU6JL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 3 of 4 RICH CREME CLEANSER
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR PEG-100 STEARATE (UNII: YD01N1999R) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR STEARYL HEPTANOATE (UNII: 2M4UGL1NCN) INGR STEARYL CAPRYLATE (UNII: 06TS6O9194) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) INGR ISOHEXADECANE (UNII: 918X1OUF1E) INGR POLYSORBATE 60 (UNII: CAL22UVI4M) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR ROYAL JELLY (UNII: L497I37F0C) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 4 of 4 ROYAL DEFY MULTI-PERFORMANCE AGE PROTECTOR BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Item Code (Source) NDC:68828-761 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) SUCROSE STEARATE (UNII: 274KW0O50M) CERIC OXIDE (UNII: 619G5K328Y) ALUMINUM OXIDE (UNII: LMI26O6933) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) CERAMIDE NP (UNII: 4370DF050B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYSORBATE 60 (UNII: CAL22UVI4M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIPROPYLENE GLYCOL (UNII: E107L85C40) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) AVOCADO OIL (UNII: 6VNO72PFC1) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) WATER (UNII: 059QF0KO0R) COCOA (UNII: D9108TZ9KG) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) SQUALANE (UNII: GW89575KF9) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SOYBEAN (UNII: L7HT8F1ZOD) SODIUM BENZOATE (UNII: OJ245FE5EU) ROYAL JELLY (UNII: L497I37F0C) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SHEA BUTTER (UNII: K49155WL9Y) OLIVE OIL (UNII: 6UYK2W1W1E) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) FARNESOL (UNII: EB41QIU6JL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-761-01 7.5 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/13/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/12/2022 ROYAL DEFY MULTI-PERFORMANCE AGE PROTECTOR BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-764 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SHEA BUTTER (UNII: K49155WL9Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE NP (UNII: 4370DF050B) CERIC OXIDE (UNII: 619G5K328Y) DIPROPYLENE GLYCOL (UNII: E107L85C40) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SOYBEAN (UNII: L7HT8F1ZOD) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) PEG-100 STEARATE (UNII: YD01N1999R) AVOCADO OIL (UNII: 6VNO72PFC1) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYSORBATE 60 (UNII: CAL22UVI4M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ROYAL JELLY (UNII: L497I37F0C) SODIUM BENZOATE (UNII: OJ245FE5EU) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) SQUALANE (UNII: GW89575KF9) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) SUCROSE STEARATE (UNII: 274KW0O50M) COCOA (UNII: D9108TZ9KG) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) FARNESOL (UNII: EB41QIU6JL) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-764-01 50 mL in 1 CARTON; Type 0: Not a Combination Product 01/12/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/12/2022 JAFRA ROYAL DEFY TRAVEL KIT
avobenzone, homosalate, octisalate, octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-762 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-762-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 01/13/2022 11/30/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 7.5 mL Part 2 1 TUBE 14 mL Part 3 1 TUBE 30 mL Part 4 1 TUBE 14 mL Part 1 of 4 ACTIVE FIRMING SERUM
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CAFFEINE (UNII: 3G6A5W338E) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) INGR HYALURONIC ACID (UNII: S270N0TRQY) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR WATER (UNII: 059QF0KO0R) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR BIS-PEG-18 METHYL ETHER DIMETHYL SILANE (UNII: OEB4R3WW9C) INGR LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR SILICON DIOXIDE (UNII: ETJ7Z6XBU4) INGR POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ROYAL JELLY (UNII: L497I37F0C) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR METHYL GLUCETH-20 (UNII: J3QD0LD11P) INGR DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) INGR ACETYL TETRAPEPTIDE-11 (UNII: 0R3VC3BV8I) INGR ACETYL TETRAPEPTIDE-9 (UNII: VMO8OOD3V0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 2 of 4 4D DEFINING HYDRATOR
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR XYLITOL (UNII: VCQ006KQ1E) INGR XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) INGR ANHYDROXYLITOL (UNII: 8XWR7NN42F) INGR OCTYLDODECANOL (UNII: 461N1O614Y) INGR OCTYLDODECYL XYLOSIDE (UNII: 8Z6VNR46QM) INGR PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) INGR AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) INGR ETHYLHEXYL PALMITATE (UNII: 2865993309) INGR CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) INGR CAFFEINE (UNII: 3G6A5W338E) INGR DEXTRAN 40 (UNII: K3R6ZDH4DU) INGR TRIFLUOROACETYL TRIPEPTIDE-2 (UNII: E6WT9V3SGO) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR ISOSORBIDE DICAPRYLATE (UNII: 0IK29C4889) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) INGR JOJOBA OIL (UNII: 724GKU717M) INGR CERAMIDE NG (UNII: C04977SRJ5) INGR PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR TRIBEHENIN (UNII: 8OC9U7TQZ0) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR ROYAL JELLY (UNII: L497I37F0C) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 3 of 4 RICH CREME CLEANSER
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR PEG-100 STEARATE (UNII: YD01N1999R) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR STEARYL HEPTANOATE (UNII: 2M4UGL1NCN) INGR STEARYL CAPRYLATE (UNII: 06TS6O9194) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) INGR ISOHEXADECANE (UNII: 918X1OUF1E) INGR POLYSORBATE 60 (UNII: CAL22UVI4M) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR ROYAL JELLY (UNII: L497I37F0C) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 4 of 4 ROYAL DEFY AGE AND POLLUTION PROTECTOR BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Item Code (Source) NDC:68828-763 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) SUCROSE STEARATE (UNII: 274KW0O50M) CERIC OXIDE (UNII: 619G5K328Y) ALUMINUM OXIDE (UNII: LMI26O6933) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) CERAMIDE NP (UNII: 4370DF050B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYSORBATE 60 (UNII: CAL22UVI4M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIPROPYLENE GLYCOL (UNII: E107L85C40) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) AVOCADO OIL (UNII: 6VNO72PFC1) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) WATER (UNII: 059QF0KO0R) COCOA (UNII: D9108TZ9KG) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) SQUALANE (UNII: GW89575KF9) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SOYBEAN (UNII: L7HT8F1ZOD) SODIUM BENZOATE (UNII: OJ245FE5EU) ROYAL JELLY (UNII: L497I37F0C) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) FARNESOL (UNII: EB41QIU6JL) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SHEA BUTTER (UNII: K49155WL9Y) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-763-01 14 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/13/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/12/2022 Labeler - Jafra cosmetics International (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-760, 68828-761, 68828-762, 68828-763, 68828-764)