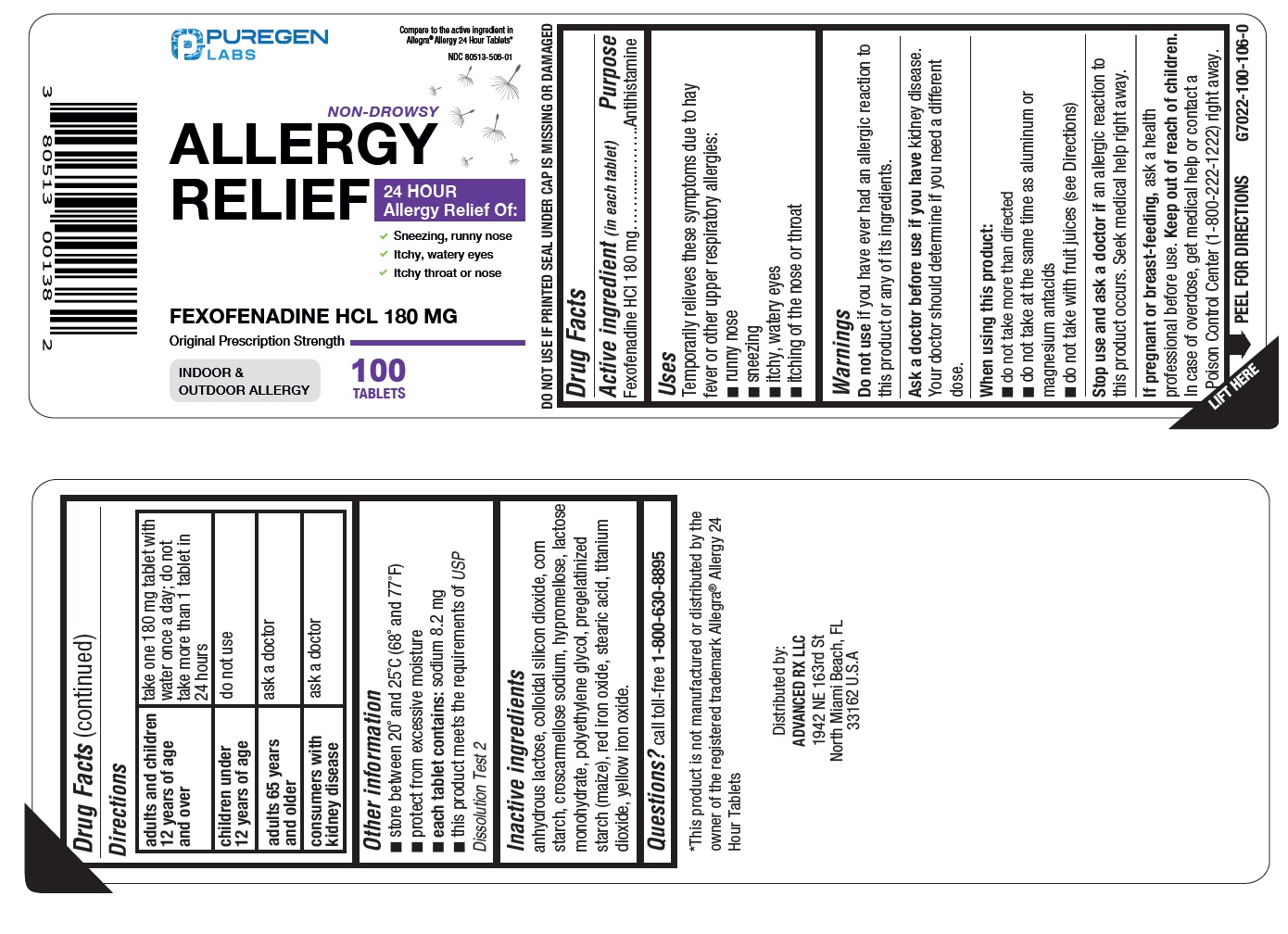
Label: FEXOFENADINE HCL tablet, film coated
- NDC Code(s): 80513-506-01
- Packager: Advanced Rx LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Fexofenadine HCl 180 mg
-
Purpose
Antihistamine
-
Uses
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - sneezing - itchy, watery eyes - itching of the nose or throat
-
Warnings
Do not use - if you have ever had an allergic reaction to this product or any of its ingredients. Ask a doctor before use if you have - kidney disease. Your doctor should determine if you need ...
-
Directions
adults and children 12 years of age and over - take one 180mg tablet with water once a day; do not take more than 1 tablet in 24 hours - children under 12 years of age - do not ...
-
Other information
store between 20° and 25°C (68° and 77°F) protect from excessive moisture - each tablet contains:sodium 8.2 mg - this product meets the requirements of - USPDissolution Test 2 - DO NOT USE ...
-
Inactive ingredientsanhydrous lactose, colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose, lactose monohydrate, polyethylene glycol, pregelatinized starch (maize), red iron oxide, stearic ...
-
Questions?
call toll free - 1-800-630-8895
-
SPL UNCLASSIFIED SECTIONDistributed by: Advanced Rx LLC - 1942 NE 163rd St North Miami Beach, FL 33162 U.S.A.
-
Package/Label Principal Display PanelNDC 80513-506-01 - Compare to the active ingredient in Allegra - ®Allergy 24 Hour Tablets - * Non-Drowsy - Allergy Relief - Fexofenadine HCL 180 mg - INDOOR & OUTDOOR ALLERGY - 24-Hour Allergy ...
-
INGREDIENTS AND APPEARANCEProduct Information