Label: AI KUNGANG- calcium carbonate liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 81768-0001-1 - Packager: TheJoenSpace Co,. LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
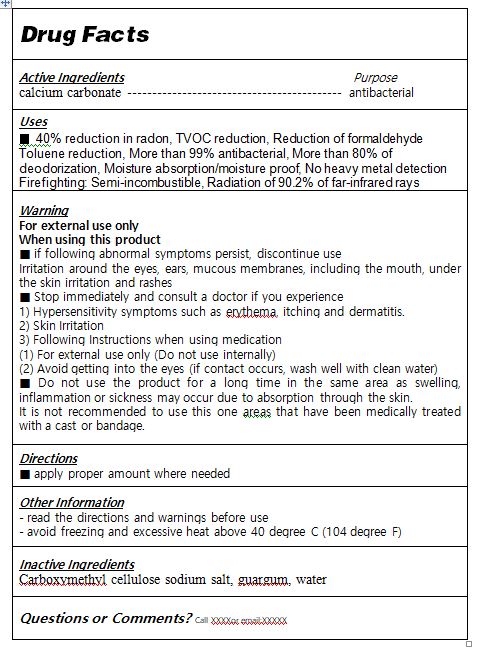
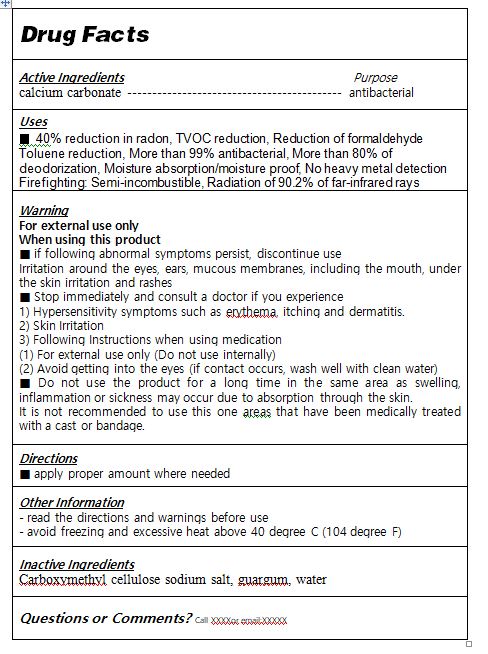
ACTIVE INGREDIENTcalcium carbonate
-
INACTIVE INGREDIENTCarboxymethyl cellulose sodium salt, guargum, water
-
PURPOSE40% reduction in radon - TVOC reduction - Reduction of formaldehyde - Toluene reduction - More than 99% antibacterial - More than 80% of deodorization - Moisture absorption/moisture ...
-
WARNINGS■ if following abnormal symptoms persist, discontinue use - Irritation around the eyes, ears, mucous membranes, including the mouth, under the skin irritation and rashes - ■ Stop immediately and ...
-
KEEP OUT OF REACH OF CHILDREN• Keep Out of Reach of Children.
-
INDICATIONS & USAGE■ apply proper amount to the skin
-
DOSAGE & ADMINISTRATIONfor external use only
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information