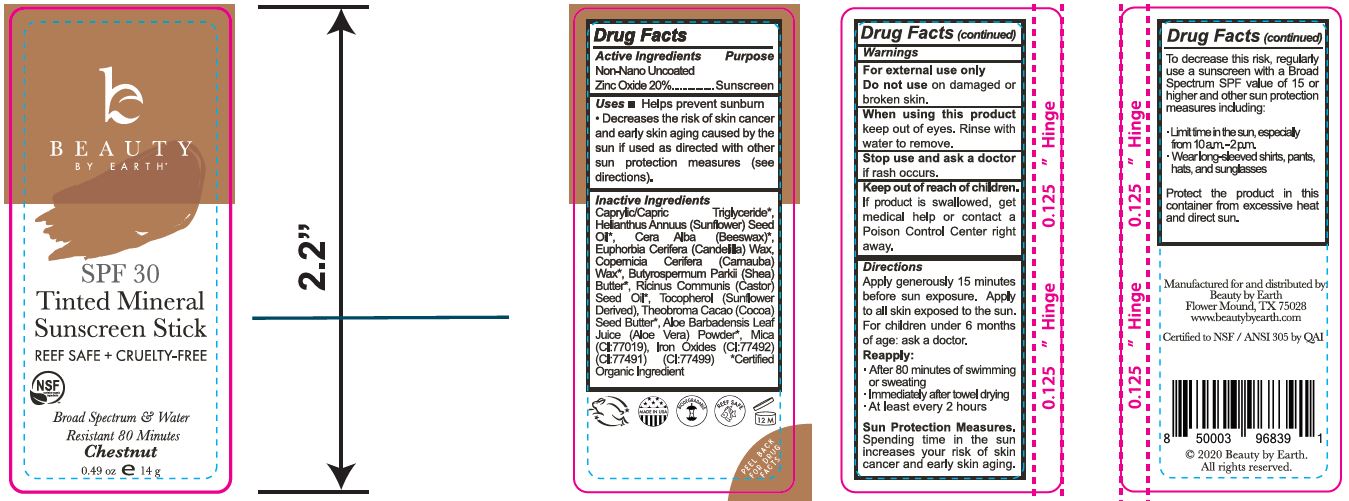
Label: TINTED MINERAL FACE SUNSCREEN STICK - CHESTNUT stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 62932-224-33 - Packager: Private Label Select Ltd CO
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)