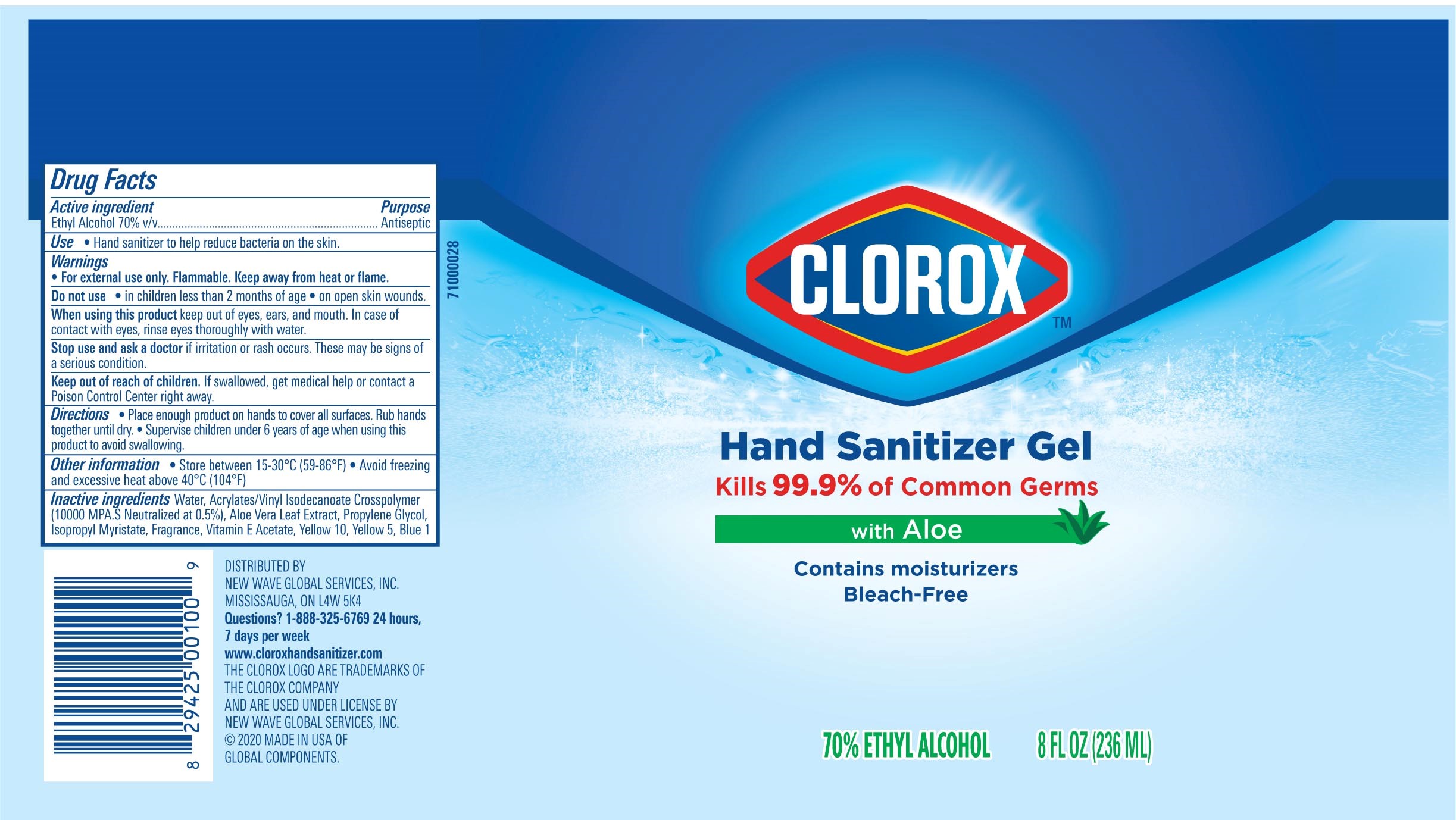
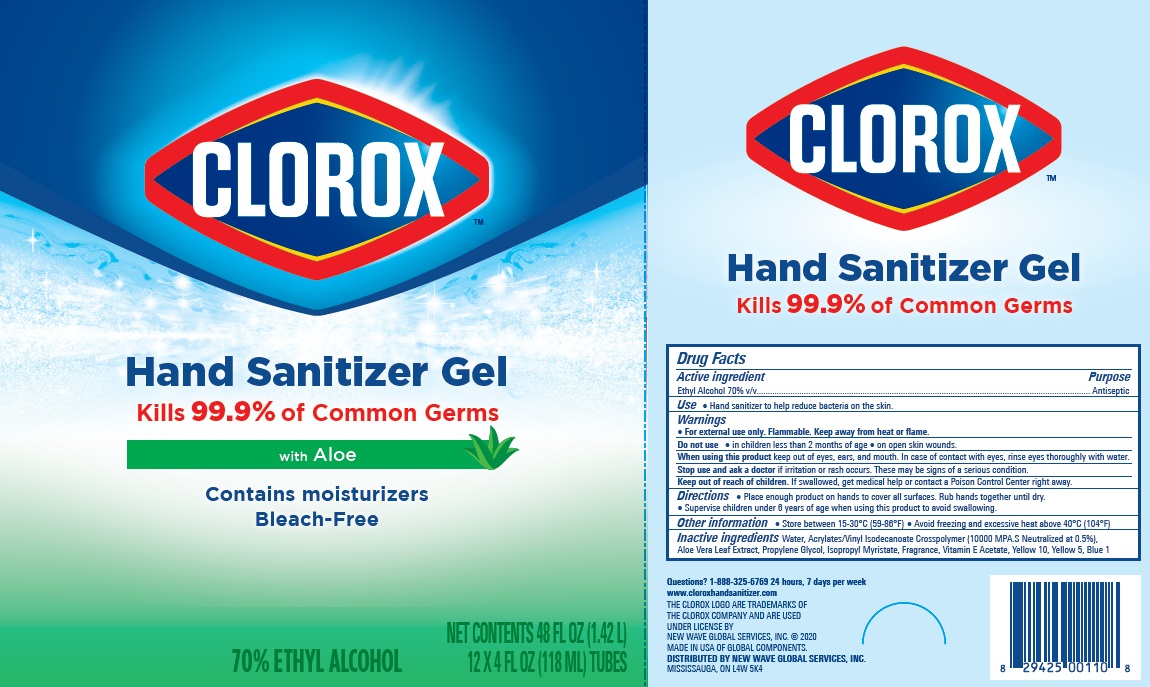
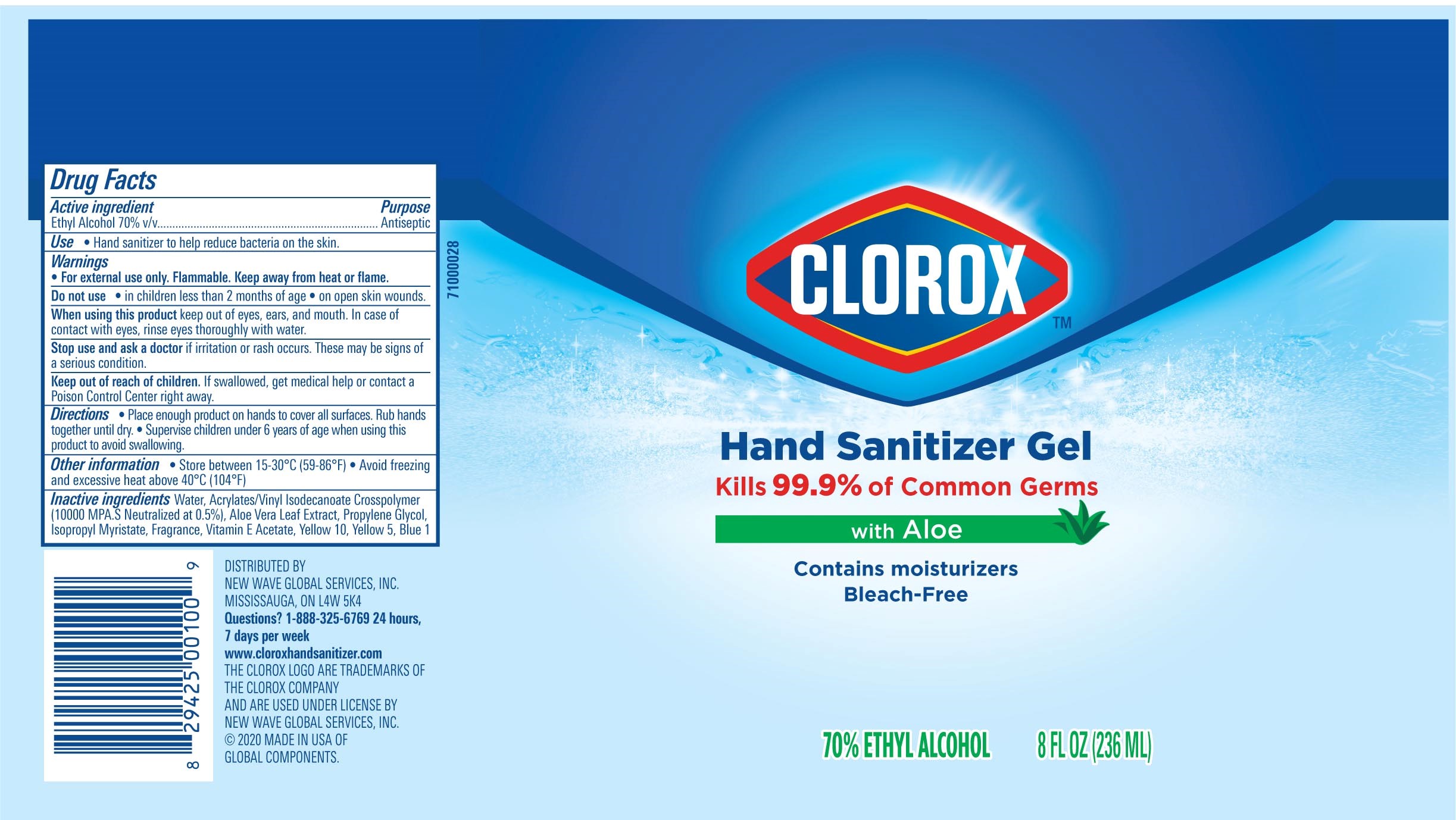
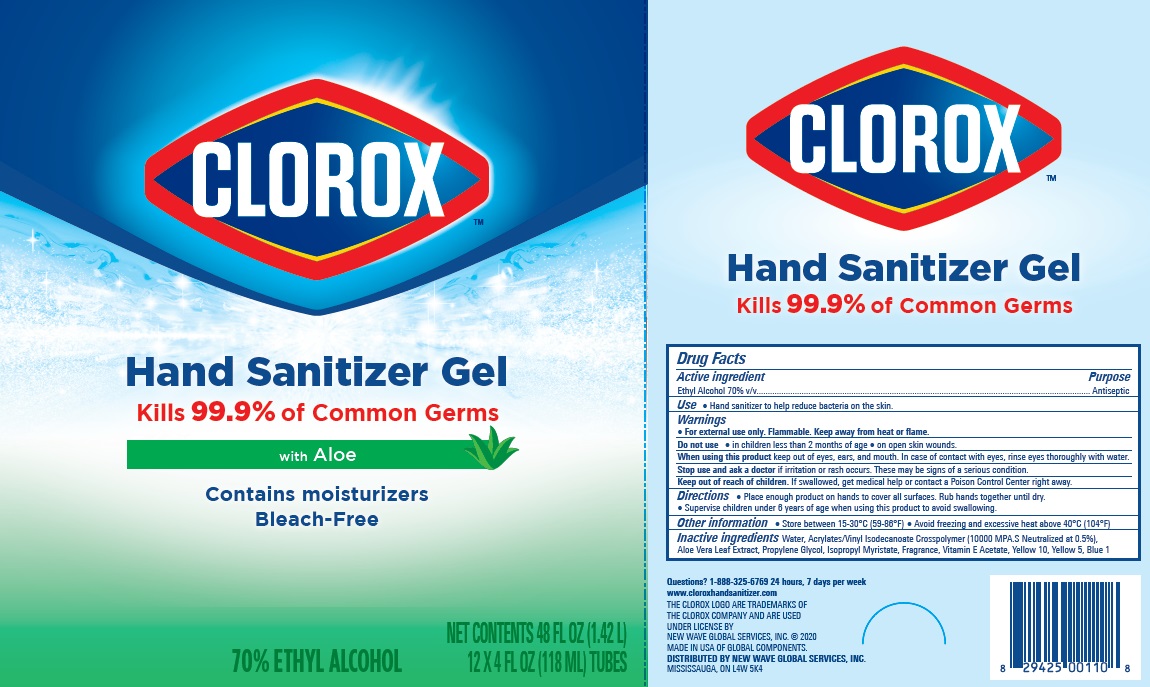
Label: CLOROX HAND SANITIZER GEL- alcohol hand sanitizer gel
-
NDC Code(s):
80714-034-01,
80714-034-02,
80714-034-03,
80714-034-04, view more80714-034-05, 80714-034-06, 80714-034-08, 80714-034-09, 80714-034-10, 80714-034-11
- Packager: New Wave Global Services Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Package Label - Principal Display Panel

clorox HS 4OZx12 Carton, NDC:80714-034-04
CLOROX HS 4OZ Tube, NDC: 80714-034-02

CLOROX HS Gallon Jug- NDC 80714-034-08

CLOROX HS Pouch-NDC 80714-034-05

CLOROX HS 24x Single Use Pouches Carton- NDC 80714-034-06

CLOROX HS 12X2oz Tube Display Carton- NDC 80714-034-03

CLOROX HS 2OZ Tube-NDC 80714-034-01

6 x 12 oz Bottles

-
INGREDIENTS AND APPEARANCE
CLOROX HAND SANITIZER GEL
alcohol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80714-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE (UNII: V5VD430YW9) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80714-034-03 12 in 1 CARTON 10/30/2020 02/28/2023 1 NDC:80714-034-01 59 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:80714-034-08 3780 mL in 1 JUG; Type 0: Not a Combination Product 01/20/2021 01/31/2025 3 NDC:80714-034-06 24 in 1 CARTON 01/20/2021 01/31/2023 3 NDC:80714-034-05 3 mL in 1 POUCH; Type 0: Not a Combination Product 4 NDC:80714-034-09 236 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/25/2021 02/29/2024 5 NDC:80714-034-11 6 in 1 CARTON 10/30/2020 03/31/2024 5 NDC:80714-034-10 354 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 6 NDC:80714-034-04 12 in 1 CARTON 10/30/2020 03/31/2024 6 NDC:80714-034-02 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/30/2020 01/31/2025 Labeler - New Wave Global Services Inc (202806733)