Label: CALQUENCE- acalabrutinib tablet, film coated
- NDC Code(s): 0310-3512-60, 0310-3512-95
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CALQUENCE safely and effectively. See full prescribing information for CALQUENCE.
CALQUENCE® (acalabrutinib) tablets, for oral use
Initial U.S. Approval: 2017RECENT MAJOR CHANGES
INDICATIONS AND USAGE
CALQUENCE is a kinase inhibitor indicated for the treatment of adult patients with:
- •
- Mantle cell lymphoma (MCL) who have received at least one prior therapy. (1.1)
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1.1, 14.1) - •
- Chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). (1.2)
DOSAGE AND ADMINISTRATION
- •
- Recommended dose is 100 mg orally approximately every 12 hours; swallow whole with water and with or without food. (2.1)
- •
- Advise patients not to chew, crush, dissolve, or cut tablets. (2.1)
- •
- Manage toxicities using treatment interruption, dose reduction, or discontinuation. (2.2)
- •
- Avoid CALQUENCE in patients with severe hepatic impairment. (8.6)
DOSAGE FORMS AND STRENGTHS
Tablets: 100 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- •
- Serious and Opportunistic Infections: Monitor for signs and symptoms of infection and treat promptly. (5.1)
- •
- Hemorrhage: Monitor for bleeding and manage appropriately. (5.2)
- •
- Cytopenias: Monitor complete blood counts regularly. (5.3)
- •
- Second Primary Malignancies: Other malignancies have occurred, including skin cancers and other solid tumors. Advise patients to use sun protection. (5.4)
- •
- Cardiac Arrhythmias: Monitor for symptoms of arrhythmias and manage. (5.5)
- •
- Hepatotoxicity, Including Drug Induced Liver Injury: Monitor hepatic function throughout treatment. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 30%) are: anemia, neutropenia, upper respiratory tract infection, thrombocytopenia, headache, diarrhea, and musculoskeletal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Mantle Cell Lymphoma
1.2 Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Dosage for Drug Interactions
2.3 Dosage Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious and Opportunistic Infections
5.2 Hemorrhage
5.3 Cytopenias
5.4 Second Primary Malignancies
5.5 Cardiac Arrhythmias
5.6 Hepatotoxicity, Including Drug-Induced Liver Injury
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on CALQUENCE
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Mantle Cell Lymphoma
14.2 Chronic Lymphocytic Leukemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Mantle Cell Lymphoma
CALQUENCE is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
This indication is approved under accelerated approval based on overall response rate [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
CALQUENCE as Monotherapy
For patients with MCL, CLL, or SLL, the recommended dosage of CALQUENCE is 100 mg taken orally approximately every 12 hours until disease progression or unacceptable toxicity.
CALQUENCE in Combination with Obinutuzumab
For patients with previously untreated CLL or SLL, the recommended dosage of CALQUENCE is 100 mg taken orally approximately every 12 hours until disease progression or unacceptable toxicity. Start CALQUENCE at Cycle 1 (each cycle is 28 days). Start obinutuzumab at Cycle 2 for a total of 6 cycles and refer to the obinutuzumab prescribing information for recommended dosing. Administer CALQUENCE prior to obinutuzumab when given on the same day.
Advise patients to swallow tablet whole with water. Advise patients not to chew, crush, dissolve, or cut the tablets. CALQUENCE may be taken with or without food. If a dose of CALQUENCE is missed by more than 3 hours, it should be skipped and the next dose should be taken at its regularly scheduled time. Extra tablets of CALQUENCE should not be taken to make up for a missed dose.
2.2 Recommended Dosage for Drug Interactions
Dosage Modifications for Use with CYP3A Inhibitors or Inducers
These are described in Table 1 [see Drug Interactions (7)].
Table 1: Recommended Dosage Modifications for Use with CYP3A Inhibitors or Inducers CYP3A
Co-administered Drug
Recommended CALQUENCE use
Inhibition
Strong CYP3A inhibitor
Avoid co-administration.
If these inhibitors will be used short-term (such as anti‑infectives for up to seven days), interrupt CALQUENCE.
After discontinuation of strong CYP3A inhibitor for at least 24 hours, resume previous dosage of CALQUENCE.
Moderate CYP3A inhibitor
Reduce the CALQUENCE 100 mg every 12 hours dosage to 100 mg once daily.
Induction
Strong CYP3A inducer
Avoid co-administration.
If co-administration is unavoidable, increase CALQUENCE dosage to 200 mg approximately every 12 hours.
2.3 Dosage Modifications for Adverse Reactions
Recommended dosage modifications of CALQUENCE for Grade 3 or greater adverse reactions are provided in Table 2.
Table 2: Recommended Dosage Modifications for Adverse Reactions Event Adverse Reaction Occurrence Dosage Modification
(Starting dose = 100 mg approximately every 12 hours)Grade 3 or greater non-hematologic toxicities,
Grade 3 thrombocytopenia with bleeding,
Grade 4 thrombocytopenia or
Grade 4 neutropenia lasting longer than 7 days
First and Second
Interrupt CALQUENCE.
Once toxicity has resolved to Grade 1 or baseline level, CALQUENCE may be resumed at 100 mg approximately every 12 hours.
Third
Interrupt CALQUENCE.
Once toxicity has resolved to Grade 1 or baseline level, CALQUENCE may be resumed at a reduced frequency of 100 mg once daily.
Fourth
Discontinue CALQUENCE.
Adverse reactions graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE).
Refer to the obinutuzumab prescribing information for management of obinutuzumab toxicities.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious and Opportunistic Infections
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients exposed to CALQUENCE in clinical trials, most often due to respiratory tract infections (11% of all patients, including pneumonia in 6%) [see Adverse Reactions (6.1)]. These infections predominantly occurred in the absence of Grade 3 or 4 neutropenia, with neutropenic infection reported in 1.9% of all patients. Opportunistic infections in recipients of CALQUENCE have included, but are not limited to, hepatitis B virus reactivation, fungal pneumonia, Pneumocystis jirovecii pneumonia, Epstein-Barr virus reactivation, cytomegalovirus, and progressive multifocal leukoencephalopathy (PML). Consider prophylaxis in patients who are at increased risk for opportunistic infections. Monitor patients for signs and symptoms of infection and treat promptly.
5.2 Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematologic malignancies treated with CALQUENCE. Major hemorrhage (serious or Grade 3 or higher bleeding or any central nervous system bleeding) occurred in 3.0% of patients, with fatal hemorrhage occurring in 0.1% of 1029 patients exposed to CALQUENCE in clinical trials. Bleeding events of any grade, excluding bruising and petechiae, occurred in 22% of patients [see Adverse Reactions (6.1)].
Use of antithrombotic agents concomitantly with CALQUENCE may further increase the risk of hemorrhage. In clinical trials, major hemorrhage occurred in 2.7% of patients taking CALQUENCE without antithrombotic agents and 3.6% of patients taking CALQUENCE with antithrombotic agents. Consider the risks and benefits of antithrombotic agents when co-administered with CALQUENCE. Monitor patients for signs of bleeding.
Consider the benefit-risk of withholding CALQUENCE for 3 to 7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
5.3 Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (23%), anemia (8%), thrombocytopenia (7%), and lymphopenia (7%), developed in patients with hematologic malignancies treated with CALQUENCE. Grade 4 neutropenia developed in 12% of patients [see Adverse Reactions (6.1)]. Monitor complete blood counts regularly during treatment. Interrupt treatment, reduce the dose, or discontinue treatment as warranted [see Dosage and Administration (2.3)].
5.4 Second Primary Malignancies
Second primary malignancies, including skin cancers and other solid tumors, occurred in 12% of 1029 patients exposed to CALQUENCE in clinical trials [see Adverse Reactions (6.1)]. The most frequent second primary malignancy was skin cancer, reported in 6% of patients. Monitor patients for skin cancers and advise protection from sun exposure.
5.5 Cardiac Arrhythmias
Serious cardiac arrhythmias have occurred in patients treated with CALQUENCE. Grade 3 atrial fibrillation or flutter occurred in 1.1% of 1029 patients treated with CALQUENCE, with all grades of atrial fibrillation or flutter reported in 4.1% of all patients [see Adverse Reactions (6.1)]. Grade 3 or higher ventricular arrhythmia events were reported in 0.9% of patients. The risk may be increased in patients with cardiac risk factors, hypertension, previous arrhythmias, and acute infection. Monitor for symptoms of arrhythmia (e.g., palpitations, dizziness, syncope, dyspnea) and manage as appropriate.
5.6 Hepatotoxicity, Including Drug-Induced Liver Injury
Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of drug-induced liver injury (DILI), has occurred in patients treated with Bruton tyrosine kinase inhibitors, including CALQUENCE.
Evaluate bilirubin and transaminases at baseline and throughout treatment with CALQUENCE. For patients who develop abnormal liver tests after CALQUENCE, monitor more frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If DILI is suspected, withhold CALQUENCE. Upon confirmation of DILI, discontinue CALQUENCE.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Serious and Opportunistic Infections [see Warnings and Precautions (5.1)]
- •
- Hemorrhage [see Warnings and Precautions (5.2)]
- •
- Cytopenias [see Warnings and Precautions (5.3)]
- •
- Second Primary Malignancies [see Warnings and Precautions (5.4)]
- •
- Cardiac Arrhythmias [see Warnings and Precautions (5.5)]
- •
- Hepatotoxicity, including DILI [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
As clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the Warnings and Precautions reflect exposure to CALQUENCE 100 mg approximately every 12 hours in 1029 patients with hematologic malignancies. Treatment includes CALQUENCE monotherapy in 820 patients in 6 trials, and CALQUENCE with obinutuzumab in 209 patients in 2 trials. Among these recipients of CALQUENCE, 88% were exposed for at least 6 months and 79% were exposed for at least one year. In this pooled safety population, adverse reactions in ≥ 30% of 1029 patients were anemia, neutropenia, upper respiratory tract infection, thrombocytopenia, headache, diarrhea, and musculoskeletal pain.
Mantle Cell Lymphoma
The safety data described in this section reflect exposure to CALQUENCE (100 mg approximately every 12 hours) in 124 patients with previously treated MCL in Trial LY-004 [see Clinical Studies (14.1)]. The median duration of treatment with CALQUENCE was 16.6 (range: 0.1 to 26.6) months. A total of 91 (73.4%) patients were treated with CALQUENCE for ≥ 6 months and 74 (59.7%) patients were treated for ≥ 1 year.
The most common adverse reactions (≥ 20%) of any grade were anemia, thrombocytopenia, headache, neutropenia, diarrhea, fatigue, myalgia, and bruising. Grade 1 severity for the non-hematologic, most common events were as follows: headache (25%), diarrhea (16%), fatigue (20%), myalgia (15%), and bruising (19%). The most common Grade ≥ 3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea.
Dose reductions and discontinuation due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively.
Tables 3 and 4 present the frequency category of adverse reactions observed in patients with MCL treated with CALQUENCE.
Table 3: Non-Hematologic Adverse Reactions in ≥ 5% (All Grades) of Patients with MCL in Trial LY-004 Body System
Adverse Reactions*CALQUENCE Monotherapy
N=124All Grades (%) Grade ≥ 3 (%) Nervous system disorders
Headache
39
1.6
Gastrointestinal disorders
Diarrhea
31
3.2
Nausea
19
0.8
Abdominal pain
15
1.6
Constipation
15
-
Vomiting
13
1.6
General disorders
Fatigue
28
0.8
Musculoskeletal and connective tissue disorders
Myalgia
21
0.8
Skin and subcutaneous tissue disorders
Bruising†
21
-
Rash‡
18
0.8
Vascular disorders
Hemorrhage§
8
0.8
Respiratory, thoracic and mediastinal disorders
Epistaxis
6
-
Table 4: Hematologic Adverse Reactions Reported in ≥ 20% of Patients with MCL in Trial LY-004 - *
- Per NCI CTCAE version 4.03; based on laboratory measurements and adverse reactions.
Hematologic
Adverse Reactions*
CALQUENCE Monotherapy
N=124
All Grades (%)
Grade ≥ 3 (%)
Hemoglobin decreased
46
10
Platelets decreased
44
12
Neutrophils decreased
36
15
Increases in creatinine to 1.5 to 3 times the upper limit of normal (ULN) occurred in 4.8% of patients.
Chronic Lymphocytic Leukemia
The safety data described below reflect exposure to CALQUENCE (100 mg approximately every 12 hours, with or without obinutuzumab) in 511 patients with CLL from two randomized controlled clinical trials [see Clinical Studies (14.2)].
The most common adverse reactions (≥ 30%) of any grade in patients with CLL were anemia, neutropenia, thrombocytopenia, headache, upper respiratory tract infection, and diarrhea.
ELEVATE-TN
The safety of CALQUENCE plus obinutuzumab (CALQUENCE+G), CALQUENCE monotherapy, and obinutuzumab plus chlorambucil (GClb) was evaluated in a randomized, multicenter, open-label, actively controlled trial in 526 patients with previously untreated CLL [see Clinical Studies (14.2)].
Patients randomized to the CALQUENCE+G arm were treated with CALQUENCE and obinutuzumab in combination for six cycles, then with CALQUENCE as monotherapy until disease progression or unacceptable toxicity. Patients initiated obinutuzumab on Day 1 of Cycle 2, continuing for a total of 6 cycles. Patient randomized to CALQUENCE monotherapy received CALQUENCE approximately every 12 hours until disease progression or unacceptable toxicity. The trial required age ≥ 65 years of age or 18 to < 65 years of age with a total Cumulative Illness Rating Scale (CIRS) > 6 or creatinine clearance of 30 to 69 mL/min, hepatic transaminases ≤ 3 times ULN and total bilirubin ≤ 1.5 times ULN, and allowed patients to receive antithrombotic agents other than warfarin or equivalent vitamin K antagonists.
During randomized treatment, the median duration of exposure to CALQUENCE in the CALQUENCE+G and CALQUENCE monotherapy arms was 27.7 months (range 0.3 to 40 months), with 95% and 92% and 89% and 86% of patients with at least 6 months and 12 months of exposure, respectively. In the obinutuzumab and chlorambucil arm the median number of cycles was 6 with 84% of patients receiving at least 6 cycles of obinutuzumab, 70% of patients received at least 6 cycles of chlorambucil. Eighty-five percent of patients in the CALQUENCE+G arm received at least 6 cycles of obinutuzumab.
In the CALQUENCE+G and CALQUENCE monotherapy arms, fatal adverse reactions that occurred in the absence of disease progression and with onset within 30 days of the last study treatment were reported in 2% for each treatment arm, most often from infection. Serious adverse reactions were reported in 39% of patients in the CALQUENCE+G arm and 32% in the CALQUENCE monotherapy arm, most often due to events of pneumonia (2.8% to 7%).
In the CALQUENCE+G arm, adverse reactions led to treatment discontinuation in 11% of patients and a dose reduction of CALQUENCE in 7% of patients. In the CALQUENCE monotherapy arm, adverse reactions led to discontinuation in 10% and dose reduction in 4% of patients.
Tables 5 and 6 present adverse reactions and laboratory abnormalities identified in the ELEVATE-TN trial.
Table 5: Common Adverse Reactions (≥ 15% Any Grade) with CALQUENCE in Patients with CLL (ELEVATE-TN) Body System
Adverse Reaction*
CALQUENCE plus Obinutuzumab
N=178CALQUENCE Monotherapy
N=179Obinutuzumab plus Chlorambucil
N=169All Grades (%) Grade ≥ 3 (%) All Grades (%) Grade ≥ 3 (%) All Grades (%) Grade ≥ 3 (%) - *
- Per NCI CTCAE version 4.03
- †
- Includes any adverse reactions involving infection or febrile neutropenia
- ‡
- Includes 3 fatal cases in the CALQUENCE plus obinutuzumab arm, 3 fatal cases in the CALQUENCE monotherapy arm and 1 fatal case in the obinutuzumab plus chlorambucil arm
- §
- Includes upper respiratory tract infection, nasopharyngitis and sinusitis
- ¶
- Includes pneumonia, lower respiratory tract infection, bronchitis, bronchiolitis, tracheitis, and lung infection
- #
- Derived from adverse reaction and laboratory data
- Þ
- Includes neutropenia, neutrophil count decreased, and related laboratory data
- ß
- Includes anemia, red blood cell count decreased, and related laboratory data
- à
- Includes thrombocytopenia, platelet count decreased, and related laboratory data
- è
- Includes lymphocytosis, lymphocyte count increased, and related laboratory data
- ð
- Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal discomfort, myalgia, neck pain, pain in extremity and spinal pain
- ø
- Includes asthenia, fatigue, and lethargy
- ý
- Includes bruise, contusion, and ecchymosis
- £
- Includes rash, dermatitis, and other related terms
- ¥
- Includes hemorrhage, hematoma, hemoptysis, hematuria, menorrhagia, hemarthrosis, and epistaxis
- Infections
- Infection†
69
22‡
65
14‡
46
13‡
- Upper respiratory tract infection§
39
2.8
35
0
17
1.2
- Lower respiratory tract infection¶
24
8
18
4.5
7
1.8
- Urinary tract infection
15
1.7
15
2.8
5
0.6
- Blood and lymphatic system disorders#
- NeutropeniaÞ
53
37
23
13
78
50
- Anemiaß
52
12
53
10
54
14
- Thrombocytopeniaà
51
12
32
3.4
61
16
- Lymphocytosisè
12
11
16
15
0.6
0.6
- Nervous system disorders
- Headache
40
1.1
39
1.1
12
0
- Dizziness
20
0
12
0
7
0
- Gastrointestinal disorders
- Diarrhea
39
4.5
35
0.6
21
1.8
- Nausea
20
0
22
0
31
0
- Musculoskeletal and connective tissue disorders
- Musculoskeletal painð
37
2.2
32
1.1
16
2.4
- Arthralgia
22
1.1
16
0.6
4.7
1.2
- General disorders and administration site conditions
- Fatigueø
34
2.2
23
1.1
24
1.2
- Skin and subcutaneous tissue disorders
- Bruisingý
- 31
- 0
- 21
- 0
- 5
- 0
- Rash£
- 26
- 2.2
- 25
- 0.6
- 9
- 0.6
- Vascular disorders
- Hemorrhage¥
20
1.7
20
1.7
6
0
Other clinically relevant adverse reactions (all grades incidence < 15%) in recipients of CALQUENCE (CALQUENCE in combination with obinutuzumab and monotherapy) included:
- •
- Neoplasms: second primary malignancy (10%), non-melanoma skin cancer (5%)
- •
- Cardiac disorders: atrial fibrillation or flutter (3.6%), hypertension (5%)
- •
- Infection: herpesvirus infection (6%)
Table 6: Select Non-Hematologic Laboratory Abnormalities (≥ 15% Any Grade), New or Worsening from Baseline in Patients Receiving CALQUENCE (ELEVATE-TN) Laboratory Abnormality*† CALQUENCE plus Obinutuzumab
N=178CALQUENCE Monotherapy
N=179Obinutuzumab plus Chlorambucil
N=169All
Grades (%)Grade ≥ 3 (%) All
Grades (%)Grade ≥ 3 (%) All
Grades (%)Grade ≥ 3 (%) Uric acid increase
29
29
22
22
37
37
ALT increase
30
7
20
1.1
36
6
AST increase
38
5
17
0.6
60
8
Bilirubin increase
13
0.6
15
0.6
11
0.6
Increases in creatinine to 1.5 to 3 times ULN occurred in 3.9% and 2.8% of patients in the CALQUENCE combination arm and monotherapy arm, respectively.
ASCEND
The safety of CALQUENCE in patients with relapsed or refractory CLL was evaluated in a randomized, open-label study (ASCEND) [see Clinical Studies (14.2)]. The trial enrolled patients with relapsed or refractory CLL after at least one prior therapy and required hepatic transaminases ≤ 2 times ULN, total bilirubin ≤ 1.5 times ULN, and an estimated creatinine clearance ≥ 30 mL/min. The trial excluded patients having an absolute neutrophil count < 500/µL, platelet count < 30,000/µL, prothrombin time or activated partial thromboplastin time > 2 times ULN, significant cardiovascular disease, or a requirement for strong CYP3A inhibitors or inducers. Patients were allowed to receive antithrombotic agents other than warfarin or equivalent vitamin K antagonist.
In ASCEND, 154 patients received CALQUENCE (100 mg approximately every 12 hours until disease progression or unacceptable toxicity), 118 received idelalisib (150 mg approximately every 12 hours until disease progression or unacceptable toxicity) with up to 8 infusions of a rituximab product, and 35 received up to 6 cycles of bendamustine and a rituximab product. The median age overall was 68 years (range: 32-90); 67% were male; 92% were white; and 88% had an ECOG performance status of 0 or 1.
In the CALQUENCE arm, serious adverse reactions occurred in 29% of patients. Serious adverse reactions in > 5% of patients who received CALQUENCE included lower respiratory tract infection (6%). Fatal adverse reactions within 30 days of the last dose of CALQUENCE occurred in 2.6% of patients, including from second primary malignancies and infection.
In recipients of CALQUENCE, permanent discontinuation due to an adverse reaction occurred in 10% of patients, most frequently due to second primary malignancies followed by infection. Adverse reactions led to dosage interruptions of CALQUENCE in 34% of patients, most often due to respiratory tract infections followed by neutropenia, and dose reduction in 3.9% of patients.
Selected adverse reactions are described in Table 7 and non-hematologic laboratory abnormalities are described in Table 8. These tables reflect exposure to CALQUENCE with median duration of 15.7 months with 94% of patients on treatment for greater than 6 months and 86% of patients on treatment for greater than 12 months. The median duration of exposure to idelalisib was 11.5 months with 72% of patients on treatment for greater than 6 months and 48% of patients on treatment for greater than 12 months. Eighty-three percent of patients completed 6 cycles of bendamustine and rituximab product.
Table 7: Common Adverse Reactions (≥ 15% Any Grade) with CALQUENCE in Patients with CLL (ASCEND) Body System
Adverse Reaction*
CALQUENCE
N=154Idelalisib plus Rituximab
Product N=118Bendamustine plus Rituximab Product
N=35All
Grades (%)Grade ≥ 3 (%) All
Grades (%)Grade ≥ 3 (%) All
Grades (%)Grade ≥ 3 (%) - *
- Per NCI CTCAE version 4.03
- †
- Includes any adverse reactions involving infection or febrile neutropenia
- ‡
- Includes 1 fatal case in the CALQUENCE monotherapy arm and 1 fatal case in the Idelalisib plus Rituximab arm
- §
- Includes upper respiratory tract infection, rhinitis and nasopharyngitis
- ¶
- Includes pneumonia, lower respiratory tract infection, bronchitis, bronchiolitis, tracheitis, and lung infection.
- #
- Derived from adverse reaction and laboratory data
- Þ
- Includes neutropenia, neutrophil count decreased, and related laboratory data
- ß
- Includes anemia, red blood cell decreased, and related laboratory data
- à
- Includes thrombocytopenia, platelet count decreased, and related laboratory data
- è
- Includes lymphocytosis, lymphocyte count increased and related laboratory data
- ð
- Includes colitis, diarrhea, and enterocolitis
- ø
- Includes hemorrhage, hematoma, hemoptysis, hematuria, menorrhagia, hemarthrosis, and epistaxis
- ý
- Includes asthenia, fatigue, and lethargy
- £
- Includes back pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal discomfort, pain in extremity, myalgia, spinal pain and bone pain
- Infections
- Infection†
- 56
- 15‡
- 65
- 28‡
- 49
- 11
- Upper respiratory tract infection§
- 29
- 1.9
- 26
- 3.4
- 17
- 2.9
- Lower respiratory tract infection¶
- 23
- 6
- 26
- 15
- 14
- 6
Blood and lymphatic system disorders#
- NeutropeniaÞ
- 48
- 23
- 79
- 53
- 80
- 40
- Anemiaß
- 47
- 15
- 45
- 8
- 57
- 17
- Thrombocytopeniaà
- 33
- 6
- 41
- 13
- 54
- 6
- Lymphocytosisè
- 26
- 19
- 23
- 18
- 2.9
- 2.9
- Nervous system disorders
- Headache
- 22
- 0.6
- 6
- 0
- 0
- 0
- Gastrointestinal disorders
- Diarrheað
- 18
- 1.3
- 49
- 25
- 14
- 0
- Vascular disorders
- Hemorrhageø
- 16
- 1.3
- 5
- 1.7
- 6
- 2.9
- General disorders
- Fatigueý
- 15
- 1.9
- 13
- 0.8
- 31
- 6
- Musculoskeletal and connective tissue disorders
- Musculoskeletal pain£
- 15
- 1.3
- 15
- 1.7
- 2.9
- 0
Other clinically relevant adverse reactions (all grades incidence < 15%) in recipients of CALQUENCE included:
- •
- Skin and subcutaneous disorders: bruising (10%), rash (9%)
- •
- Neoplasms: second primary malignancy (12%), non-melanoma skin cancer (6%)
- •
- Musculoskeletal and connective tissue disorders: arthralgia (8%)
- •
- Cardiac disorders: atrial fibrillation or flutter (5%), hypertension (3.2%)
- •
- Infection: herpesvirus infection (4.5%)
Table 8: Select Non-Hematologic Laboratory Abnormalities (≥ 10% Any Grade), New or Worsening from Baseline in Patients Receiving CALQUENCE (ASCEND) Laboratory Abnormality* CALQUENCE
N=154Idelalisib plus Rituximab Product
N=118Bendamustine plus Rituximab Product
N=35All
Grades
(%)Grade ≥ 3 (%) All
Grades
(%)Grade ≥ 3 (%) All
Grades (%)Grade ≥ 3 (%) - *
- Excludes electrolytes
Uric acid increase
15
15
11
11
23
23
ALT increase
15
1.9
59
23
26
2.9
AST increase
13
0.6
48
13
31
2.9
Bilirubin increase
13
1.3
16
1.7
26
11
Per NCI CTCAE version 5
Increases in creatinine to 1.5 to 3 times ULN occurred in 1.3% of patients who received CALQUENCE.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of CALQUENCE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Cardiac disorders: ventricular arrhythmias
- •
- Hepatobiliary disorder: Drug induced liver injury (DILI)
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on CALQUENCE
Strong CYP3A Inhibitors
Clinical Effect
Co-administration of CALQUENCE with a strong CYP3A inhibitor increased acalabrutinib plasma concentrations [see Clinical Pharmacology (12.3)]. Increased acalabrutinib concentrations may result in increased toxicity.
Prevention or Management
Avoid co-administration of CALQUENCE with strong CYP3A inhibitors. Alternatively, if the inhibitor will be used short-term, interrupt CALQUENCE [see Dosage and Administration (2.2)].
Moderate CYP3A Inhibitors
Clinical Effect
Co-administration of CALQUENCE with a moderate CYP3A inhibitor may increase acalabrutinib plasma concentration [see Clinical Pharmacology (12.3)]. Increased acalabrutinib concentrations may result in increased toxicity.
Prevention or Management
Reduce the dosage of CALQUENCE when co-administered with a moderate CYP3A inhibitor [see Dosage and Administration (2.2)].
Strong CYP3A Inducers
Clinical Effect
Co-administration of CALQUENCE with a strong CYP3A inducer decreased acalabrutinib plasma concentration [see Clinical Pharmacology (12.3)]. Decreased acalabrutinib concentrations may reduce CALQUENCE activity.
Prevention or Management
Avoid co-administration of CALQUENCE with strong CYP3A inducers. If co-administration is unavoidable, increase the dosage of CALQUENCE [see Dosage and Administration (2.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals, CALQUENCE may cause fetal harm and dystocia when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of acalabrutinib to animals during organogenesis resulted in dystocia in rats and reduced fetal growth in rabbits at maternal exposures (AUC) 2 times exposures in patients at the recommended dose of 100 mg approximately every 12 hours (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In a combined fertility and embryo-fetal development study in female rats, acalabrutinib was administered orally at doses up to 200 mg/kg/day starting 14 days prior to mating through gestational day [GD] 17. No effects on embryo-fetal development and survival were observed. The AUC at 200 mg/kg/day in pregnant rats was approximately 9 times the AUC in patients at the recommended dose of 100 mg approximately every 12 hours. The presence of acalabrutinib and its active metabolite were confirmed in fetal rat plasma.
In an embryo-fetal development study in rabbits, pregnant animals were administered acalabrutinib orally at doses up to 200 mg/kg/day during the period of organogenesis (from GD 6-18). Administration of acalabrutinib at doses ≥ 100 mg/kg/day produced maternal toxicity and 100 mg/kg/day resulted in decreased fetal body weights and delayed skeletal ossification. The AUC at 100 mg/kg/day in pregnant rabbits was approximately 2 times the AUC in patients at 100 mg approximately every 12 hours.
In a pre- and postnatal development study in rats, acalabrutinib was administered orally to pregnant animals during organogenesis, parturition and lactation, at doses of 50, 100, and 150 mg/kg/day. Dystocia (prolonged or difficult labor) and mortality of offspring were observed at doses ≥ 100 mg/kg/day. The AUC at 100 mg/kg/day in pregnant rats was approximately 2 times the AUC in patients at 100 mg approximately every 12 hours. Underdeveloped renal papilla was also observed in F1 generation offspring at 150 mg/kg/day with an AUC approximately 5 times the AUC in patients at 100 mg approximately every 12 hours.
8.2 Lactation
Risk Summary
No data are available regarding the presence of acalabrutinib or its active metabolite in human milk, its effects on the breastfed child, or on milk production. Acalabrutinib and its active metabolite were present in the milk of lactating rats. Due to the potential for adverse reactions in a breastfed child from CALQUENCE, advise lactating women not to breastfeed while taking CALQUENCE and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
CALQUENCE may cause embryo-fetal harm and dystocia when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating CALQUENCE therapy.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with CALQUENCE and for 1 week following the last dose of CALQUENCE. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be informed of the potential hazard to a fetus.
8.4 Pediatric Use
The safety and efficacy of CALQUENCE in pediatric patients have not been established.
8.5 Geriatric Use
Of the 929 patients with CLL or MCL in clinical trials of CALQUENCE, 68% were 65 years of age or older, and 24% were 75 years of age or older. Among patients 65 years of age or older, 59% had Grade 3 or higher adverse reactions and 39% had serious adverse reactions. Among patients younger than age 65, 45% had Grade 3 or higher adverse reactions and 25% had serious adverse reactions. No clinically relevant differences in efficacy were observed between patients ≥ 65 years and younger.
8.6 Hepatic Impairment
Avoid use of CALQUENCE in patients with severe hepatic impairment (Child-Pugh class C). No dosage adjustment of CALQUENCE is recommended in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment. The safety of CALQUENCE has not been evaluated in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
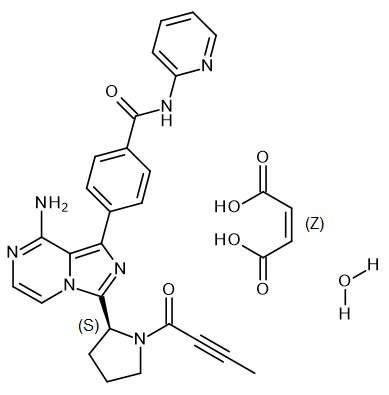
11 DESCRIPTION
CALQUENCE (acalabrutinib) is a kinase inhibitor. The molecular formula for acalabrutinib maleate is C26H23N7O2•C4H4O4.H2O, and the molecular weight is 599.59. The chemical name is 4-{8-Amino-3-[(2S)-1-(but-2-ynoyl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide (2Z)-2-butenedioic acid hydrate.
The chemical structure of acalabrutinib is shown below:
Acalabrutinib maleate is a white to pale brown powder with pH-dependent solubility. It is freely soluble in water at pH values below 3 and practically insoluble at pH values above 6.
CALQUENCE tablets are for oral administration. Each tablet contains 100 mg of acalabrutinib (equivalent to 129 mg of acalabrutinib maleate). Inactive ingredients in the tablet core are low-substituted hydroxypropyl cellulose, mannitol, microcrystalline cellulose, and sodium stearyl fumarate. The tablet coating consists of copovidone, ferric oxide yellow, ferric oxide red, hypromellose, medium-chain triglycerides, polyethylene glycol 3350, purified water and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Acalabrutinib is a small-molecule inhibitor of Bruton tyrosine kinase (BTK). Acalabrutinib and its active metabolite, ACP-5862, form a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B cell antigen receptor (BCR) and cytokine receptor pathways. In B cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion. In nonclinical studies, acalabrutinib inhibited BTK‑mediated activation of downstream signaling proteins CD86 and CD69 and inhibited malignant B-cell proliferation and tumor growth in mouse xenograft models.
12.2 Pharmacodynamics
In patients with B-cell malignancies dosed with acalabrutinib 100 mg approximately every 12 hours, median steady state BTK occupancy of ≥ 95% in peripheral blood was maintained over 12 hours, resulting in inactivation of BTK throughout the recommended dosing interval.
Cardiac Electrophysiology
At a dose 4 times the approved recommended dosage, CALQUENCE does not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
Acalabrutinib and its active metabolite, ACP-5862, exposures increase proportionally with dose across a dose range of 75 to 250 mg (0.75 to 2.5 times the approved recommended single dosage) in patients with B-cell malignancies. At the recommended dose of 100 mg twice daily, the geometric mean (% coefficient of variation [CV]) daily area under the plasma drug concentration over time curve (AUC24h) and maximum plasma concentration (Cmax) for acalabrutinib were 1843 (38%) ng•h/mL and 563 (29%) ng/mL, respectively, and for ACP-5862 were 3947 (43%) ng•h/mL and 451 (52%) ng/mL, respectively.
Absorption
The geometric mean absolute bioavailability of acalabrutinib was 25%. Median (min, max) time to peak plasma concentration (Tmax) of acalabrutinib and its active metabolite, ACP-5862 were 0.5 (0.2, 3.0) hours and 0.75 (0.5, 4.0) hours, respectively.
Effect of Food
In healthy subjects, administration of a single 100 mg dose of acalabrutinib with a high-fat, high-calorie meal (approximately 918 calories, 59 grams carbohydrate, 59 grams fat, and 39 grams protein) did not affect the mean AUC as compared to dosing under fasted conditions. Resulting Cmax decreased by 54% and Tmax was delayed 1-2 hours.
Distribution
The geometric mean (% CV) steady-state volume of distribution (Vss) of acalabrutinib and its active metabolite, ACP-5862 was approximately 101 (52%) L and 67 (32%) L, respectively. Human plasma protein of acalabrutinib and its active metabolite, ACP-5862, were 97.5% and 98.6%, respectively. The mean blood-to-plasma ratio of acalabrutinib and its active metabolite, ACP-5862, was 0.8 and 0.7, respectively.
Elimination
The geometric mean (% CV) terminal elimination half-life (t1/2) of acalabrutinib and its active metabolite, ACP-5862, were 1.4 (50%) hours and 6.4 (37%) hours, respectively. The geometric mean (%CV) apparent oral clearance (CL/F) of acalabrutinib and its active metabolite, ACP-5862, were 71 (35%) L/hr and 13 (42%) L/hr, respectively.
Metabolism
Acalabrutinib is predominantly metabolized by CYP3A enzymes, and to a minor extent, by glutathione conjugation and amide hydrolysis, based on in vitro studies. ACP-5862 was identified as the major active metabolite in plasma with a geometric mean exposure (AUC) that was approximately 2- to 3-fold higher than the exposure of acalabrutinib. ACP-5862 is approximately 50% less potent than acalabrutinib with regard to BTK inhibition.
Excretion
Following administration of a single 100 mg radiolabeled acalabrutinib dose in healthy subjects, 84% of the dose was recovered in the feces (< 2% unchanged) and 12% of the dose was recovered in the urine (< 2% unchanged).
Specific Populations
There were no clinically significant differences in the pharmacokinetics of acalabrutinib and its active metabolite, ACP‑5862, based on age (32 to 90 years), sex, race (Caucasian, African American), body weight (40 to 149 kg), or mild to moderate renal impairment (eGFR ≥ 30 mL/min/1.73m2 and eGFR < 89 mL/min/1.73m2, as estimated by MDRD (modification of diet in renal disease equation)). The effect of severe renal impairment (eGFR < 29 mL/min/1.73m2, MDRD) or renal impairment requiring dialysis on the pharmacokinetics of acalabrutinib is unknown.
Patients with Hepatic Impairment
The AUC of acalabrutinib increased 1.9-fold in subjects with mild hepatic impairment (Child-Pugh class A), 1.5-fold in subjects with moderate hepatic impairment (Child-Pugh class B) and 5.3-fold in subjects with severe hepatic impairment (Child-Pugh class C) compared to subjects with normal liver function. No clinically relevant PK difference in ACP-5862 was observed in subjects with severe hepatic impairment (Child-Pugh Class C) compared to subjects with normal liver function. No clinically relevant PK differences in acalabrutinib and ACP-5862 were observed in patients with mild or moderate hepatic impairment (total bilirubin less and equal to upper limit of normal [ULN] and AST greater than ULN, or total bilirubin greater than ULN and any AST) relative to patients with normal hepatic function (total bilirubin and AST within ULN).
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Strong CYP3A Inhibitors: Co-administration of acalabrutinib with itraconazole (strong CYP3A inhibitor) increase acalabrutinib Cmax by 3.9-fold and AUC by 5.1-fold in healthy subjects.
Moderate CYP3A Inhibitors: Co-administration of acalabrutinib with erythromycin (moderate CYP3A inhibitor), fluconazole (moderate CYP3A inhibitor), diltiazem (moderate CYP3A inhibitor) is predicted to increase acalabrutinib Cmax and AUC by approximately 2- to 3-fold.
Strong CYP3A Inducers: Co-administration of acalabrutinib with rifampin (strong CYP3A inducer) decreased acalabrutinib Cmax by 68% and AUC by 77% in healthy subjects.
Acid-Reducing Agents: No clinically significant differences in the pharmacokinetics of acalabrutinib were observed when co-administered with rabeprazole (proton pump inhibitor).
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Acalabrutinib is an inhibitor of CYP3A4/5, CYP2C8 and CYP2C9, but not CYP1A2, CYP2B6, CYP2C19, or CYP2D6. Acalabrutinib’s active metabolite, ACP-5862, is an inhibitor of CYP2C8, CYP2C9 and CYP2C19, but not CYP1A2, CYP2B6, CYP2D6, or CYP3A4/5. Acalabrutinib is an inducer of CYP1A2, CYP2B6, and CYP3A4. Acalabrutinib’s active metabolite, ACP-5862, is an inducer of CYP3A4.
Uridine diphosphate (UDP)-glucuronosyl transferase (UGT) Enzymes: Acalabrutinib and its active metabolite, ACP‑5862, are not inhibitors of UGT1A1 or UGT2B7.
Transporter System: Acalabrutinib is an inhibitor of breast cancer resistance protein (BCRP), but not multidrug and toxin extrusion protein 1 (MATE1). Acalabrutinib’s active metabolite, ACP-5862, is an inhibitor of MATE1, but not BCRP. Acalabrutinib and its active metabolite, ACP-5862, are not inhibitors of P-glycoprotein (P-gp), organic anion transporter (OAT) 1, OAT3, organic cation transporter 2 (OCT2), organic anion transporting polypeptide (OATP) 1B1, OATP1B3, or MATE2-K.
Acalabrutinib and its active metabolite, ACP-5862, are substrates of P-gp and BCRP. Acalabrutinib is not a substrate of OAT1, OAT3, OCT2, OATP1B1, or OATP1B3. Acalabrutinib’s active metabolite, ACP-5862, is not a substrate of OATP1B1 or OATP1B3.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with acalabrutinib.
Acalabrutinib was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberration assay or in an in vivo rat bone marrow micronucleus assay.
In a fertility study in rats, there were no effects of acalabrutinib on fertility in male rats at exposures 11 times, or in female rats at exposures 9 times, the AUC observed in patients at the recommended dose of 100 mg twice daily.
-
14 CLINICAL STUDIES
14.1 Mantle Cell Lymphoma
The efficacy of CALQUENCE was based upon Trial LY-004 titled “An Open-label, Phase 2 Study of ACP-196 in Subjects with Mantle Cell Lymphoma” (NCT02213926). Trial LY-004 enrolled a total of 124 patients with MCL who had received at least one prior therapy.
The median age was 68 (range 42 to 90) years, 80% were male, and 74% were Caucasian. At baseline, 93% of patients had an ECOG performance status of 0 or 1. The median time since diagnosis was 46.3 months and the median number of prior treatments was 2 (range 1 to 5), including 18% with prior stem cell transplant. Patients who received prior treatment with BTK inhibitors were excluded. The most common prior regimens were CHOP-based (52%) and ARA-C (34%). At baseline, 37% of patients had at least one tumor with a longest diameter ≥ 5 cm, 73% had extra nodal involvement including 51% with bone marrow involvement. The simplified Mantle Cell Lymphoma International Prognostic Index (MIPI) score (which includes age, ECOG score, and baseline lactate dehydrogenase and white cell count) was intermediate in 44% and high in 17% of patients.
CALQUENCE was administered orally at 100 mg approximately every 12 hours until disease progression or unacceptable toxicity. The median dose intensity was 98.5%. The major efficacy outcome of Trial LY-004 was overall response rate and the median follow-up was 15.2 months.
Table 9: Efficacy Results in Patients with MCL in Trial LY-004 - *
- Per 2014 Lugano Classification.
Investigator Assessed
N=124
Independent Review Committee (IRC) Assessed
N=124
Overall Response Rate (ORR)*
ORR (%) [95% CI]
81 [73, 87]
80 [72, 87]
Complete Response (%) [95% CI]
40 [31, 49]
40 [31, 49]
Partial Response (%) [95% CI]
41 [32, 50]
40 [32, 50]
Duration of Response (DoR)
Median DoR in months [range]
NE [1+ to 20+]
NE [0+ to 20+]
CI= Confidence Interval; NE=Not Estimable; + indicates censored observations.
The median time to best response was 1.9 months.
Lymphocytosis
Upon initiation of CALQUENCE, a temporary increase in lymphocyte counts (defined as absolute lymphocyte count increased ≥ 50% from baseline and a post-baseline assessment ≥ 5 x 109/L) in 31.5% of patients in Trial LY-004. The median time to onset of lymphocytosis was 1.1 weeks, and the median duration of lymphocytosis was 6.7 weeks.
14.2 Chronic Lymphocytic Leukemia
The efficacy of CALQUENCE in patients with CLL was demonstrated in two randomized, controlled trials. The indication for CALQUENCE includes patients with SLL because it is the same disease.
ELEVATE-TN
The efficacy of CALQUENCE was evaluated in the ELEVATE-TN trial, a randomized, multicenter, open-label, actively controlled, 3 arm trial of CALQUENCE in combination with obinutuzumab, CALQUENCE monotherapy, and obinutuzumab in combination with chlorambucil in 535 patients with previously untreated chronic lymphocytic leukemia (NCT02475681). Patients 65 years of age or older or between 18 and 65 years of age with a total Cumulative Illness Rating Scale (CIRS) > 6 or creatinine clearance of 30 to 69 mL/min were enrolled. The trial also required hepatic transaminases ≤ 3 times upper limit of normal (ULN) and total bilirubin ≤ 1.5 times ULN, and excluded patients with Richter’s transformation.
Patients were randomized in a 1:1:1 ratio into 3 arms to receive:
- •
- CALQUENCE plus obinutuzumab (CALQUENCE+G): CALQUENCE 100 mg was administered approximately every 12 hours starting on Cycle 1 Day 1 until disease progression or unacceptable toxicity. Obinutuzumab was administered starting on Cycle 2 Day 1 for a maximum of 6 treatment cycles. Obinutuzumab 1,000 mg was administered on Days 1 and 2 (100 mg on Day 1 and 900 mg on Day 2), 8 and 15 of Cycle 2 followed by 1,000 mg on Day 1 of Cycles 3 up to 7. Each cycle was 28 days.
- •
- CALQUENCE monotherapy: CALQUENCE 100 mg was administered approximately every 12 hours until disease progression or unacceptable toxicity.
- •
- Obinutuzumab plus chlorambucil (GClb): Obinutuzumab and chlorambucil were administered for a maximum of 6 treatment cycles. Obinutuzumab 1,000 mg was administered intravenously on Days 1 and 2 (100 mg on Day 1 and 900 mg on Day 2), 8 and 15 of Cycle 1 followed by 1,000 mg on Day 1 of Cycles 2 to 6. Chlorambucil 0.5 mg/kg was administered orally on Days 1 and 15 of Cycles 1 to 6. Each cycle was 28 days.
Randomization was stratified by 17p deletion mutation status, ECOG performance status (0 or 1 versus 2), and geographic region. A total of 535 patients were randomized, 179 to CALQUENCE+G, 179 to CALQUENCE monotherapy, and 177 to GClb. The overall median age was 70 years (range: 41 to 91 years), 47% had Rai stage III or IV disease, 14% had 17p deletion or TP53 mutation, 63% of patients had an unmutated IGVH, and 18% had 11q deletion. Baseline demographic and disease characteristics were similar between treatment arms.
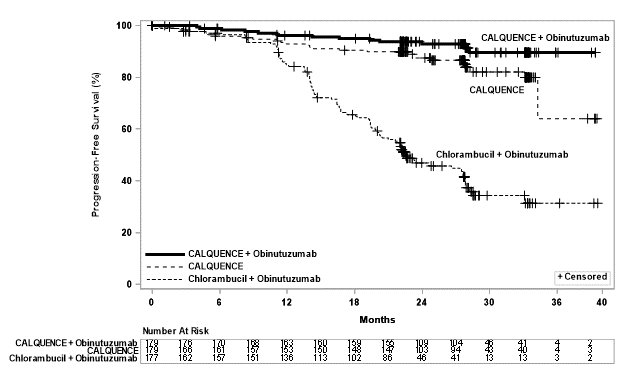
Efficacy was based on progression-free survival (PFS) as assessed by an Independent Review Committee (IRC). The median duration of follow-up was 28.3 months (range: 0.0 to 40.8 months). Efficacy results are presented in Table 10. The Kaplan-Meier curves for PFS are shown in Figure 1.
Table 10: Efficacy Results per IRC in Patients with CLL ‒ ITT population (ELEVATE-TN) - *
- Per 2008 International Workshop on CLL (IWCLL) criteria.
- †
- Kaplan-Meier estimate.
- ‡
- Based on a stratified Cox-Proportional-Hazards model. Both hazard ratios are compared with the obinutuzumab and chlorambucil arm.
- §
- Based on a stratified log-rank test, with an alpha level of 0.012 derived from alpha spending function by the O’Brien-Fleming method.
- ¶
- Based on a stratified Cochran–Mantel–Haenszel test, for the comparison with the obinutuzumab and chlorambucil arm.
CALQUENCE plus Obinutuzumab
N=179
CALQUENCE Monotherapy
N=179
Obinutuzumab plus Chlorambucil
N=177
Progression-Free Survival*
- Number of events (%)
14 (8)
26 (15)
93 (53)
- PD, n (%)
9 (5)
20 (11)
82 (46)
- Death events, n (%)
5 (3)
6 (3)
11 (6)
- Median (95% CI), months†
NE
NE (34, NE)
22.6 (20, 28)
- HR‡ (95% CI)
0.10 (0.06, 0.17)
0.20 (0.13, 0.30)
-
- p-value§
< 0.0001
< 0.0001
-
Overall Response Rate* (CR + CRi + nPR + PR)
- ORR, n (%)
168 (94)
153 (86)
139 (79)
- (95% CI)
(89, 97)
(80, 90)
(72, 84)
- p-value¶
< 0.0001
0.0763
-
- CR, n (%)
23 (13)
1 (1)
8 (5)
- CRi, n (%)
1 (1)
0
0
- nPR, n (%)
1 (1)
2 (1)
3 (2)
- PR, n (%)
143 (80)
150 (84)
128 (72)
ITT=intent-to-treat; CI=confidence interval; HR=hazard ratio; NE=not estimable; CR=complete response; CRi=complete response with incomplete blood count recovery; nPR=nodular partial response; PR=partial response.
Figure 1: Kaplan-Meier Curve of IRC-Assessed PFS in Patients with CLL in ELEVATE-TN
With a median follow-up of 28.3 months, median overall survival was not reached in any arm, with fewer than 10% of patients experiencing an event.
ASCEND
The efficacy of CALQUENCE in patients with relapsed or refractory CLL was based upon a multicenter, randomized, open-label trial (ASCEND; NCT02970318). The trial enrolled 310 patients with relapsed or refractory CLL after at least 1 prior systemic therapy. The trial excluded patients with transformed disease, prolymphocytic leukemia, or previous treatment with venetoclax, a Bruton tyrosine kinase inhibitor, or a phosphoinositide-3 kinase inhibitor.
Patients were randomized in a 1:1 ratio to receive either:
- •
- CALQUENCE 100 mg approximately every 12 hours until disease progression or unacceptable toxicity, or
- •
- Investigator’s choice:
- ∘
- Idelalisib plus a rituximab product (IR): Idelalisib 150 mg orally approximately every 12 hours until disease progression or unacceptable toxicity, in combination with 8 infusions of a rituximab product (375 mg/m2 intravenously on Day 1 of Cycle 1, followed by 500 mg/m2 every 2 weeks for 4 doses and then every 4 weeks for 3 doses), with a 28-day cycle length.
- ∘
- Bendamustine plus a rituximab product (BR): Bendamustine 70 mg/m2 intravenously (Day 1 and 2 of each 28-day cycle), in combination with a rituximab product (375 mg/m2 intravenously on Day 1 of Cycle 1, then 500 mg/m2 on Day 1 of subsequent cycles), for up to 6 cycles.
Randomization was stratified by 17p deletion mutation status, ECOG performance status (0 or 1 versus 2), and number of prior therapies (1 to 3 versus ≥ 4). Of 310 patients total, 155 were assigned to CALQUENCE monotherapy, 119 to IR, and 36 to BR. The median age overall was 67 years (range: 32 to 90 years), 42% had Rai stage III or IV disease, 28% had 17p deletion or TP53 mutation, 78% of patients had an unmutated IGVH, and 27% had a 11q deletion. The CALQUENCE arm had a median of 1 prior therapy (range: 1 to 8), with 47% having at least 2 prior therapies. The investigator’s choice arm had a median of 2 prior therapies (range: 1 to 10), with 57% having at least 2 prior therapies.
In the CALQUENCE arm, the median treatment duration was 15.7 months, with 94% of patients treated for at least 6 months and 86% of patients treated for at least 1 year. In the investigator’s choice arm, the median treatment duration was 8.4 months, with 59% of patients treated for at least 6 months and 37% treated for at least 1 year.
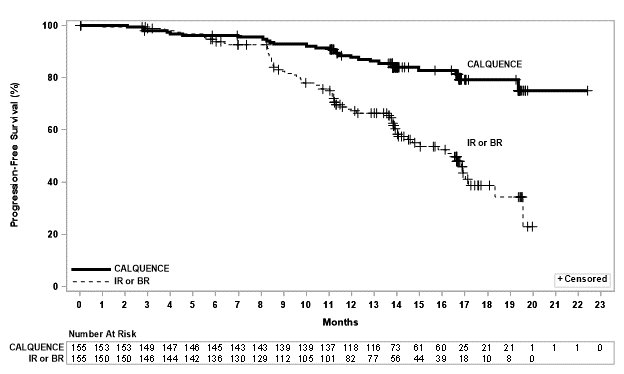
Efficacy was based on PFS as assessed by an IRC, with a median follow-up of 16.1 months (range 0.03 to 22.4 months). Efficacy results are presented in Table 11. The Kaplan-Meier curve for PFS is shown in Figure 2. There was no statistically significant difference in overall response rates between the two treatment arms.
Table 11: Efficacy Results per IRC in Patients with Relapsed or Refractory CLL – ITT Population (ASCEND) CALQUENCE Monotherapy
N=155Investigator’s Choice of Idelalisib + Rituximab Product or Bendamustine + Rituximab Product
N=155- *
- Per 2008 IWCLL criteria.
- †
- Kaplan-Meier estimate.
- ‡
- Based on a stratified Cox-Proportional-Hazards model.
- §
- Based on a stratified Log-rank test. The pre-specified type I error rate (α) for this interim analysis is 0.012 derived from a Lan-DeMets alpha spending function with O’Brien-Fleming boundary.
- ¶
- Through a hierarchical testing procedure, the difference in ORR was not statistically significant, based on a Cochran-Mantel-Haenszel test with adjustment for randomization stratification factors.
Progression-Free Survival*
- Number of events, n (%)
27 (17)
68 (44)
- Disease progression, n
19
59
- Death, n
8
9
- Median (95% CI), months†
NE (NE, NE)
16.5 (14.0, 17.1)
- HR (95% CI)‡
0.31 (0.20, 0.49)
- P-value§
< 0.0001
- ORR, n (%)¶
126 (81)
117 (75)
- (95% CI)
(74, 87)
(68, 82)
- CR, n (%)
0
2 (1)
- CRi, n (%)
0
0
- nPR, n (%)
0
0
- PR, n (%)
126 (81)
115 (74)
- ITT=intent-to-treat; CI=confidence interval; HR=hazard ratio; NE=not estimable; CR=complete response; CRi=complete response with incomplete blood count recovery; nPR=nodular partial response; PR=partial response
Figure 2: Kaplan-Meier Curve of IRC-Assessed PFS in Patients with CLL in ASCEND
With a median follow-up of 16.1 months, median overall survival was not reached in either arm, with fewer than 11% of patients experiencing an event.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Pack Size
Contents
NDC Number
60-count bottle
Bottle containing 60 tablets with a child-resistant closure
100 mg, orange, oval, biconvex tablet, with debossment ‘ACA100’ on one side and plain on the reverse
0310-3512-60
Storage
Store at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F-86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Serious and Opportunistic Infections
Inform patients of the possibility of serious infection and to report signs or symptoms suggestive of infection [see Warnings and Precautions (5.1)].
Hemorrhage
Inform patients to report signs or symptoms of bleeding. Inform patients that CALQUENCE may need to be interrupted for major surgeries [see Warnings and Precautions (5.2)].
Cytopenias
Inform patients that they will need periodic blood tests to check blood counts during treatment with CALQUENCE [see Warnings and Precautions (5.3)].
Second Primary Malignancies
Inform patients that other malignancies have been reported in patients who have been treated with CALQUENCE, including skin cancer and other solid tumors. Advise patients to use sun protection [see Warnings and Precautions (5.4)].
Cardiac Arrhythmias
Counsel patients to report any signs of palpitations, dizziness, fainting, chest discomfort, and shortness of breath [see Warnings and Precautions (5.5)].
Hepatotoxicity, including Drug Induced Liver Injury:
Inform patients that liver problems, including drug-induced liver injury and abnormalities in liver tests, may develop during CALQUENCE treatment. Advise patients to contact their healthcare provider immediately if they experience abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.6)].
Pregnancy Complication
CALQUENCE may cause fetal harm and dystocia. Advise women to use effective contraception during treatment and for 1 week after the last dose of CALQUENCE [see Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with CALQUENCE and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Dosing Instructions
Instruct patients to take CALQUENCE orally twice daily, about 12 hours apart. CALQUENCE may be taken with or without food. Advise patients that CALQUENCE tablets should be swallowed whole with a glass of water, without chewing, crushing, dissolving, or cutting [see Dosage and Administration (2.1)].
Missed Dose
Advise patients that if they miss a dose of CALQUENCE, they may still take it up to 3 hours after the time they would normally take it. If more than 3 hours have elapsed, they should be instructed to skip that dose and take their next dose of CALQUENCE at the usual time. Warn patients they should not take extra tablets to make up for the dose that they missed [see Dosage and Administration (2.1)].
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant medications, including over‑the‑counter medications, vitamins and herbal products [see Drug Interactions (7)].
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
CALQUENCE is a registered trademark of the AstraZeneca group of companies. ©AstraZeneca 2022
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
CALQUENCE® (KAL-kwens)
(acalabrutinib)
tablets
What is CALQUENCE?
CALQUENCE is a prescription medicine used to treat adults with:
- •
- Mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer.
- •
- Chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
It is not known if CALQUENCE is safe and effective in children.
Before taking CALQUENCE, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have had recent surgery or plan to have surgery. Your healthcare provider may stop CALQUENCE for any planned medical, surgical, or dental procedure.
- •
- have bleeding problems.
- •
- have or had heart rhythm problems.
- •
- have an infection.
- •
- have or had liver problems, including hepatitis B virus (HBV) infection.
- •
- are pregnant or plan to become pregnant. CALQUENCE may harm your unborn baby and problems during childbirth (dystocia).
- o
- If you are able to become pregnant, your healthcare provider may do a pregnancy test before you start treatment with CALQUENCE
- o
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with CALQUENCE and for 1 week after the last dose of CALQUENCE.
- •
- are breastfeeding or plan to breastfeed. It is not known if CALQUENCE passes into your breast milk. Do not breastfeed during treatment with CALQUENCE and for 2 weeks after your last dose of CALQUENCE.
Tell your healthcare provider about all the medicines you take, including prescription and over‑the‑counter medicines, vitamins, and herbal supplements. Taking CALQUENCE with certain other medications may affect how CALQUENCE works and can cause side effects. Especially tell your healthcare provider if you take a blood thinner medicine.
How should I take CALQUENCE?
- •
- Take CALQUENCE exactly as your healthcare provider tells you to take it.
- •
- Do not change your dose or stop taking CALQUENCE unless your healthcare provider tells you to.
- •
- Your healthcare provider may tell you to decrease your dose, temporarily stop, or completely stop taking CALQUENCE if you develop certain side effects.
- •
- Do not switch (interchange) your CALQUENCE tablets with CALQUENCE capsules.
- •
- Take CALQUENCE 2 times a day (about 12 hours apart).
- •
- Take CALQUENCE with or without food.
- •
- Swallow CALQUENCE tablets whole with a glass of water. Do not chew, crush, dissolve, or cut tablets.
- •
- If you miss a dose of CALQUENCE, take it as soon as you remember. If it is more than 3 hours past your usual dosing time, skip the missed dose and take your next dose of CALQUENCE at your regularly scheduled time. Do not take an extra dose to make up for a missed dose.
What are the possible side effects of CALQUENCE?
CALQUENCE may cause serious side effects, including:
- •
- Serious infections can happen during treatment with CALQUENCE and may lead to death. Your healthcare provider may prescribe certain medicines if you have an increased risk of getting infections. Tell your healthcare provider right away if you have any signs or symptoms of an infection, including fever, chills, or flu-like symptoms.
- •
-
Bleeding problems (hemorrhage) can happen during treatment with CALQUENCE and can be serious and may lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs or symptoms of bleeding, including:
- ∘
- blood in your stools or black stools (looks like tar)
- ∘
- pink or brown urine
- ∘
- unexpected bleeding, or bleeding that is severe or you cannot control
- ∘
- vomit blood or vomit that looks like coffee grounds
- ∘
- cough up blood or blood clots
- ∘
- dizziness
- ∘
- weakness
- ∘
- confusion
- ∘
- changes in your speech
- ∘
- headache that lasts a long time
- ∘
- bruising or red or purple skin marks
- •
- Decrease in blood cell counts. Decreased blood counts (white blood cells, platelets, and red blood cells) are common with CALQUENCE, but can also be severe. Your healthcare provider should do blood tests to check your blood counts regularly during treatment with CALQUENCE.
- •
- Second primary cancers. New cancers have happened in people during treatment with CALQUENCE, including cancers of the skin or other organs. Your healthcare provider will check you for skin cancers during treatment with CALQUENCE. Use sun protection when you are outside in sunlight.
- •
-
Heart rhythm problems (cardiac arrhythmias) have happened in people treated with CALQUENCE, which may be serious or lead to death. Tell your healthcare provider if you have any of the following signs or symptoms:
- ∘
- fast or irregular heartbeat
- ∘
- dizziness
- ∘
- feeling faint
- ∘
- chest discomfort
- ∘
- shortness of breath
- •
- Liver problems. Liver problems can happen during treatment with CALQUENCE, which may be severe or life-threatening, or lead to death. Contact your healthcare provider if you experience stomach pain or discomfort, urine of dark color or yellowing of your skin. Your healthcare provider will request tests to monitor your liver function during treatment with CALQUENCE
The most common side effects of CALQUENCE include:
- •
- headache
- •
- diarrhea
- •
- muscle and joint pain
- •
- upper respiratory tract infection
- •
- bruising
These are not all of the possible side effects of CALQUENCE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
How should I store CALQUENCE?
- •
- Store CALQUENCE at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep CALQUENCE and all medicines out of the reach of children.
General information about the safe and effective use of CALQUENCE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CALQUENCE for a condition for which it was not prescribed. Do not give CALQUENCE to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about CALQUENCE that is written for health professionals.
What are the ingredients in CALQUENCE?
Active ingredient: acalabrutinib
Inactive ingredients:
Tablet core: low-substituted hydroxypropyl cellulose, mannitol, microcrystalline cellulose, and sodium stearyl fumarate.
Tablet coating: copovidone, ferric oxide yellow, ferric oxide red, hypromellose, medium-chain triglycerides, polyethylene glycol 3350, purified water, and titanium dioxide.
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
CALQUENCE is a registered trademark of the AstraZeneca group of companies. ©AstraZeneca 2022
For more information, go to www.CALQUENCE.com or call 1-800-236-9933.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 6/2024
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALQUENCE
acalabrutinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0310-3512 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACALABRUTINIB (UNII: I42748ELQW) (ACALABRUTINIB - UNII:I42748ELQW) ACALABRUTINIB 100 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) MANNITOL (UNII: 3OWL53L36A) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color ORANGE Score no score Shape OVAL (biconvex) Size 13mm Flavor Imprint Code ACA100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0310-3512-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/04/2022 2 NDC:0310-3512-95 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/04/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216387 08/04/2022 Labeler - AstraZeneca Pharmaceuticals LP (054743190) Registrant - AstraZeneca PLC (230790719)