Label: TADALAFIL- tadalafil tablet, film coated
- NDC Code(s): 72865-104-30, 72865-106-30, 72865-107-30, 72865-108-30
- Packager: XLCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TADALAFIL TABLETS safely and effectively. See full prescribing information for TADALAFIL TABLETS. TADALAFIL tablets, for oral use ...These highlights do not include all the information needed to use TADALAFIL TABLETS safely and effectively. See full prescribing information for TADALAFIL TABLETS.
TADALAFIL tablets, for oral use
Initial U.S. Approval: 2003INDICATIONS AND USAGE
Tadalafil is a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of:
• erectile dysfunction (ED) ( 1.1)
• the signs and symptoms of benign prostatic hyperplasia (BPH) ( 1.2)
• ED and the signs and symptoms of BPH (ED/BPH) ( 1.3)
If tadalafil tablet is used with finasteride to initiate BPH treatment, such use is recommended for up to 26 weeks ( 1.4).DOSAGE AND ADMINISTRATION
• Tadalafil tablet for use as needed:
• ED: Starting dose: 10 mg as needed prior to sexual activity.
Increase to 20 mg or decrease to 5 mg based upon efficacy/tolerability. Improves erectile function compared to placebo up to 36 hours post dose. Not to be taken more than once per day (2.1).
• Tadalafil tablet for once daily use:
• ED: 2.5 mg taken once daily, without regard to timing of sexual activity. May increase to 5 mg based upon efficacy and tolerability ( 2.2).
• BPH: 5 mg, taken at approximately the same time every day ( 2.3)
• ED and BPH: 5 mg, taken at approximately the same time every day ( 2.3, 2.4)
• Tadalafil tablets may be taken without regard to food ( 2.5).
DOSAGE FORMS AND STRENGTHS
Tablets: 2.5 mg, 5 mg, 10 mg, 20 mg ( 3).
CONTRAINDICATIONS
• Administration of tadalafil tablets to patients using any form of organic nitrate is contraindicated. Tadalafil tablets was shown to potentiate the hypotensive effect of nitrates ( 4.1).
• History of known serious hypersensitivity reaction to tadalafil tablets or ADCIRCA ®( 4.2).
• Administration with guanylate cyclase (GC) stimulators, such as riociguat ( 4.3).
WARNINGS AND PRECAUTIONS
• Patients should not use tadalafil if sex is inadvisable due to cardiovascular status ( 5.1).
• Use of tadalafil with alpha-blockers, antihypertensives or substantial amounts of alcohol (≥5 units) may lead to hypotension ( 5.6, 5.9).
• Tadalafil is not recommended in combination with alpha-blockers for the treatment of BPH because efficacy of the combination has not been adequately studied and because of the risk of blood pressure lowering. Caution is advised when tadalafil tablet is used as a treatment for ED in men taking alpha-blockers. ( 2.7, 5.6, 7.1, 12.2)
• Patients should seek emergency treatment if an erection lasts >4 hours. Use tadalafil with caution in patients predisposed to priapism ( 5.3).
• Patients should stop tadalafil and seek medical care if a sudden loss of vision occurs in one or both eyes, which could be a sign of non-arteritic anterior ischemic optic neuropathy (NAION). Tadalafil tablets should be used with caution, and only when the anticipated benefits outweigh the risks, in patients with a history of NAION. Patients with a "crowded" optic disc may also be at an increased risk of NAION ( 5.4, 6.2).
• Patients should stop tadalafil and seek prompt medical attention in the event of sudden decrease or loss of hearing ( 5.5).
• Prior to initiating treatment with tadalafil tablets for BPH, consideration should be given to other urological conditions that may cause similar symptoms ( 5.14).
ADVERSE REACTIONS
Most common adverse reactions (≥2%) include headache, dyspepsia, back pain, myalgia, nasal congestion, flushing, and pain in limb ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact XLCare Pharmaceuticals, Inc. at 1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
• Tadalafil can potentiate the hypotensive effects of nitrates, alpha-blockers, antihypertensives or alcohol ( 7.1).
• CYP3A4 inhibitors (e.g. ketoconazole, ritonavir) increase tadalafil exposure ( 2.7, 5.10, 7.2) requiring dose adjustment:
• Tadalafil for use as needed: no more than 10 mg every 72 hours
• Tadalafil for once daily use: dose not to exceed 2.5 mg
• CYP3A4 inducers (e.g. rifampin) decrease tadalafil exposure ( 7.2).
USE IN SPECIFIC POPULATIONS
Hepatic Impairment ( 2.6, 5.8, 8.6):
• Mild or Moderate: Dosage adjustment may be needed.
• Severe: Use is not recommended.
Renal Impairment ( 2.6, 5.7, 8.7):
• Patients with creatinine clearance 30 to 50 mL/min: Dosage adjustment may be needed.
• Patients with creatinine clearance less than 30 mL/min or on hemodialysis: For use as needed: Dose should not exceed 5 mg every 72 hours. Once daily use is not recommended.See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Erectile Dysfunction
1.2 Benign Prostatic Hyperplasia
1.3 Erectile Dysfunction and Benign Prostatic Hyperplasia
1.4 Limitation of Use
2 DOSAGE AND ADMINISTRATION
2.1 Tadalafil Tablets for Use as Needed for Erectile Dysfunction
2.2 Tadalafil Tablets for Once Daily Use for Erectile Dysfunction
2.3 Tadalafil Tablets for Once Daily Use for Benign Prostatic Hyperplasia
2.4 Tadalafil Tablets for Once Daily Use for Erectile Dysfunction and Benign Prostatic Hyperplasia
2.5 Use with Food
2.6 Use in Specific Populations
2.7 Concomitant Medications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Nitrates
4.2 Hypersensitivity Reactions
4.3 Concomitant Guanylate Cyclase (GC) Stimulators
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular
5.2 Potential for Drug Interactions When Taking Tadalafil for Once Daily Use
5.3 Prolonged Erection
5.4 Effects on the Eye
5.5 Sudden Hearing Loss
5.6 Alpha-blockers and Antihypertensives
5.7 Renal Impairment
5.8 Hepatic Impairment
5.9 Alcohol
5.10 Concomitant Use of Potent Inhibitors of Cytochrome P450 3A4 (CYP3A4)
5.11 Combination With Other PDE5 Inhibitors or Erectile Dysfunction Therapies
5.12 Effects on Bleeding
5.13 Counseling Patients About Sexually Transmitted Diseases
5.14 Consideration of Other Urological Conditions Prior to Initiating Treatment for BPH
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Potential for Pharmacodynamic Interactions with Tadalafil
7.2 Potential for Other Drugs to Affect Tadalafil
7.3 Potential for Tadalafil to Affect Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Pharmacology and/or Toxicology
14 CLINICAL STUDIES
14.1 Tadalafil for Use as Needed for ED
14.2 Tadalafil for Once Daily Use for ED
14.3 Tadalafil 5 mg for Once Daily Use for Benign Prostatic Hyperplasia (BPH)
14.4 Tadalafil 5 mg for Once Daily Use for ED and BPH
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
17.1 Nitrates
17.2 Guanylate Cyclase (GC) Stimulators
17.3 Cardiovascular Considerations
17.4 Concomitant Use with Drugs Which Lower Blood Pressure
17.5 Potential for Drug Interactions When Taking Tadalafil Tablets for Once Daily Use
17.6 Priapism
17.7 Sudden Loss of Vision
17.8 Sudden Hearing Loss
17.9 Alcohol
17.10 Sexually Transmitted Disease
17.11 Recommended Administration
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Erectile Dysfunction - Tadalafil tablet is indicated for the treatment of erectile dysfunction (ED). 1.2 Benign Prostatic Hyperplasia - Tadalafil tablet is indicated for the treatment of ...
1.1 Erectile Dysfunction
Tadalafil tablet is indicated for the treatment of erectile dysfunction (ED).
1.2 Benign Prostatic Hyperplasia
Tadalafil tablet is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH).
1.3 Erectile Dysfunction and Benign Prostatic Hyperplasia
Tadalafil tablet is indicated for the treatment of ED and the signs and symptoms of BPH (ED/BPH).
Close1.4 Limitation of Use
If tadalafil tablet is used with finasteride to initiate BPH treatment, such use is recommended for up to 26 weeks because the incremental benefit of tadalafil tablets decreases from 4 weeks until 26 weeks, and the incremental benefit of tadalafil tablets beyond 26 weeks is unknown [see Clinical Studies ( 14.3)].
-
2 DOSAGE AND ADMINISTRATION2.1 Tadalafil Tablets for Use as Needed for Erectile Dysfunction - Do not split tadalafil tablets; entire dose should be taken. • The recommended starting dose of tadalafil tablets for ...
2.1 Tadalafil Tablets for Use as Needed for Erectile Dysfunction
Do not split tadalafil tablets; entire dose should be taken.
• The recommended starting dose of tadalafil tablets for use as needed in most patients is 10 mg, taken prior to anticipated sexual activity.
• The dose may be increased to 20 mg or decreased to 5 mg, based on individual efficacy and tolerability. The maximum recommended dosing frequency is once per day in most patients.
• Tadalafil tablets for use as needed was shown to improve erectile function compared to placebo up to 36 hours following dosing. Therefore, when advising patients on optimal use of tadalafil tablets, this should be taken into consideration.2.2 Tadalafil Tablets for Once Daily Use for Erectile Dysfunction
• The recommended starting dose of tadalafil tablets for once daily use is 2.5 mg, taken at approximately the same time every day, without regard to timing of sexual activity.
• The tadalafil tablets dose for once daily use may be increased to 5 mg, based on individual efficacy and tolerability.2.3 Tadalafil Tablets for Once Daily Use for Benign Prostatic Hyperplasia
• The recommended dose of tadalafil tablets for once daily use is 5 mg, taken at approximately the same time every day.
• When therapy for BPH is initiated with tadalafil tablets and finasteride, the recommended dose of tadalafil tablets for once daily use is 5 mg, taken at approximately the same time every day for up to 26 weeks.2.4 Tadalafil Tablets for Once Daily Use for Erectile Dysfunction and Benign Prostatic Hyperplasia
The recommended dose of tadalafil tablets for once daily use is 5 mg, taken at approximately the same time every day, without regard to timing of sexual activity.
2.6 Use in Specific Populations
Renal Impairment
Tadalafil Tablets for Use as Needed
• Creatinine clearance 30 to 50 mL/min: A starting dose of 5 mg not more than once per day is recommended, and the maximum dose is 10 mg not more than once in every 48 hours.
• Creatinine clearance less than 30 mL/min or on hemodialysis: The maximum dose is 5 mg not more than once in every 72 hours [see Warnings and Precautions ( 5.7) and Use in Specific Populations ( 8.7)].
Tadalafil Tablets for Once Daily Use
Erectile Dysfunction
• Creatinine clearance less than 30 mL/min or on hemodialysis: Tadalafil tablets for once daily use is not recommended [ see Warnings and Precautions ( 5.7) and Use in Specific Populations ( 8.7)].
Benign Prostatic Hyperplasia and Erectile Dysfunction/Benign Prostatic Hyperplasia
• Creatinine clearance 30 to 50 mL/min: A starting dose of 2.5 mg is recommended. An increase to 5 mg may be considered based on individual response.
• Creatinine clearance less than 30 mL/min or on hemodialysis: Tadalafil tablets for once daily use is not recommended [see Warnings and Precautions ( 5.7) and Use in Specific Populations ( 8.7)].
Hepatic Impairment
Tadalafil Tablets for Use as Needed
• Mild or moderate (Child Pugh Class A or B): The dose should not exceed 10 mg once per day. The use of tadalafil tablets once per day has not been extensively evaluated in patients with hepatic impairment and therefore, caution is advised.
• Severe (Child Pugh Class C): The use of tadalafil tablets is not recommended [see Warnings and Precautions ( 5.8) and Use in Specific Populations ( 8.6)].
Tadalafil Tablets for Once Daily Use
• Mild or moderate (Child Pugh Class A or B): Tadalafil tablets for once daily use has not been extensively evaluated in patients with hepatic impairment. Therefore, caution is advised if tadalafil tablets for once daily use is prescribed to these patients.
• Severe (Child Pugh Class C): The use of tadalafil tablets is not recommended [see Warnings and Precautions (5.8) and Use in Specific Populations (8.6)].
Close2.7 Concomitant Medications
Nitrates
Concomitant use of nitrates in any form is contraindicated [see Contraindications ( 4.1)].
Alpha-Blockers
ED— When tadalafil tablet is coadministered with an alpha-blocker in patients being treated for ED, patients should be stable on alpha-blocker therapy prior to initiating treatment, and tadalafil tablets should be initiated at the lowest recommended dose [see Warnings and Precautions ( 5.6), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.2)].
BPH— Tadalafil tablet is not recommended for use in combination with alpha-blockers for the treatment of BPH [see Warnings and Precautions ( 5.6), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.2)].
CYP3A4 Inhibitors
Tadalafil Tablets for Use as Needed— For patients taking concomitant potent inhibitors of CYP3A4, such as ketoconazole or ritonavir, the maximum recommended dose of tadalafil tablets is 10 mg, not to exceed once every 72 hours [see Warnings and Precautions ( 5.10) and Drug Interactions ( 7.2)].
Tadalafil Tablets for Once Daily Use — For patients taking concomitant potent inhibitors of CYP3A4, such as ketoconazole or ritonavir, the maximum recommended dose is 2.5 mg [see Warnings and Precautions ( 5.10) and Drug Interactions ( 7.2)]. -
3 DOSAGE FORMS AND STRENGTHS• Tadalafil tablets USP, 2.5 mg are blue color, round shaped, biconvex, film-coated tablets debossed with '122' on one side and 'E' on the other side. • Tadalafil tablets USP, 5 mg are white ...
• Tadalafil tablets USP, 2.5 mg are blue color, round shaped, biconvex, film-coated tablets debossed with '122' on one side and 'E' on the other side.
• Tadalafil tablets USP, 5 mg are white color, round shaped, biconvex, film-coated tablets debossed with '123' on one side and 'E' on the other side.
• Tadalafil tablets USP, 10 mg are white color, capsule shaped, biconvex, film-coated tablets debossed with '124' on one side and 'E' on the other side.
• Tadalafil tablets USP, 20 mg are white color, capsule shaped, biconvex, film-coated tablets debossed with '125' on one side and 'E' on the other side.
Close -
4 CONTRAINDICATIONS4.1 Nitrates - Administration of tadalafil tablets to patients who are using any form of organic nitrate, either regularly and/or intermittently, is contraindicated. In clinical pharmacology ...
4.1 Nitrates
Administration of tadalafil tablets to patients who are using any form of organic nitrate, either regularly and/or intermittently, is contraindicated. In clinical pharmacology studies, tadalafil tablets was shown to potentiate the hypotensive effect of nitrates [see Clinical Pharmacology ( 12.2)].
4.2 Hypersensitivity Reactions
Tadalafil tablet is contraindicated in patients with a known serious hypersensitivity to tadalafil (tadalafil tablets or ADCIRCA ®). Hypersensitivity reactions have been reported, including Stevens-Johnson syndrome and exfoliative dermatitis [see Adverse Reactions ( 6.2)].
Close4.3 Concomitant Guanylate Cyclase (GC) Stimulators
Do not use tadalafil tablets in patients who are using a GC stimulator, such as riociguat. PDE5 inhibitors, including tadalafil tablets, may potentiate the hypotensive effects of GC stimulators.
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular - Evaluation of erectile dysfunction and BPH should include an appropriate medical assessment to identify potential underlying causes, as well as treatment options ...
5.1 Cardiovascular
Evaluation of erectile dysfunction and BPH should include an appropriate medical assessment to identify potential underlying causes, as well as treatment options.
Before prescribing tadalafil, it is important to note the following:
Physicians should consider the cardiovascular status of their patients, since there is a degree of cardiac risk associated with sexual activity. Therefore, treatments for erectile dysfunction, including tadalafil, should not be used in men for whom sexual activity is inadvisable as a result of their underlying cardiovascular status. Patients who experience symptoms upon initiation of sexual activity should be advised to refrain from further sexual activity and seek immediate medical attention.
Physicians should discuss with patients the appropriate action in the event that they experience anginal chest pain requiring nitroglycerin following intake of tadalafil. In such a patient, who has taken tadalafil, where nitrate administration is deemed medically necessary for a life-threatening situation, at least 48 hours should have elapsed after the last dose of tadalafil before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring. Therefore, patients who experience anginal chest pain after taking tadalafil should seek immediate medical attention [see Contraindications ( 4.1) and Patient Counseling Information ( 17.1)].
Patients with left ventricular outflow obstruction, (e.g., aortic stenosis and idiopathic hypertrophic subaortic stenosis) can be sensitive to the action of vasodilators, including PDE5 inhibitors.
The following groups of patients with cardiovascular disease were not included in clinical safety and efficacy trials for tadalafil, and therefore until further information is available, tadalafil is not recommended for the following groups of patients:
• myocardial infarction within the last 90 days
• unstable angina or angina occurring during sexual intercourse
• New York Heart Association Class 2 or greater heart failure in the last 6 months
• uncontrolled arrhythmias, hypotension (<90/50 mm Hg), or uncontrolled hypertension
• stroke within the last 6 months.
As with other PDE5 inhibitors, tadalafil has mild systemic vasodilatory properties that may result in transient decreases in blood pressure. In a clinical pharmacology study, tadalafil 20 mg resulted in a mean maximal decrease in supine blood pressure, relative to placebo, of 1.6/0.8 mm Hg in healthy subjects [see Clinical Pharmacology ( 12.2)]. While this effect should not be of consequence in most patients, prior to prescribing tadalafil, physicians should carefully consider whether their patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects. Patients with severely impaired autonomic control of blood pressure may be particularly sensitive to the actions of vasodilators, including PDE5 inhibitors.5.2 Potential for Drug Interactions When Taking Tadalafil for Once Daily Use
Physicians should be aware that tadalafil for once daily use provides continuous plasma tadalafil levels and should consider this when evaluating the potential for interactions with medications (e.g., nitrates, alpha-blockers, anti-hypertensives and potent inhibitors of CYP3A4) and with substantial consumption of alcohol [see Drug Interactions ( 7.1, 7.2, 7.3)].
5.3 Prolonged Erection
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 hours, whether painful or not, should seek emergency medical attention.
Tadalafil should be used with caution in patients who have conditions that might predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia), or in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie's disease).5.4 Effects on the Eye
Physicians should advise patients to stop use of all phosphodiesterase type 5 (PDE5) inhibitors, including tadalafil, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition and a cause of decreased vision, including permanent loss of vision, that has been reported rarely postmarketing in temporal association with the use of all PDE5 inhibitors. Based on published literature, the annual incidence of NAION is 2.5 to 11.8 cases per 100,000 in males aged ≥50.
An observational case-crossover study evaluated the risk of NAION when PDE5 inhibitor use, as a class, occurred immediately before NAION onset (within 5 half-lives), compared to PDE5 inhibitor use in a prior time period. The results suggest an approximate 2-fold increase in the risk of NAION, with a risk estimate of 2.15 (95% CI 1.06, 4.34). A similar study reported a consistent result, with a risk estimate of 2.27 (95% CI 0.99, 5.20). Other risk factors for NAION, such as the presence of "crowded" optic disc, may have contributed to the occurrence of NAION in these studies.
Neither the rare postmarketing reports, nor the association of PDE5 inhibitor use and NAION in the observational studies, substantiate a causal relationship between PDE5 inhibitor use and NAION [see Adverse Reactions ( 6.2)].
Physicians should consider whether their patients with underlying NAION risk factors could be adversely affected by use of PDE5 inhibitors. Individuals who have already experienced NAION are at increased risk of NAION recurrence. Therefore, PDE5 inhibitors, including tadalafil, should be used with caution in these patients and only when the anticipated benefits outweigh the risks. Individuals with "crowded" optic disc are also considered at greater risk for NAION compared to the general population; however, evidence is insufficient to support screening of prospective users of PDE5 inhibitors, including tadalafil, for this uncommon condition.
Patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials, and use in these patients is not recommended.5.5 Sudden Hearing Loss
Physicians should advise patients to stop taking PDE5 inhibitors, including tadalafil, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including tadalafil. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions ( 6.1, 6.2)].
5.6 Alpha-blockers and Antihypertensives
Physicians should discuss with patients the potential for tadalafil to augment the blood-pressure-lowering effect of alpha-blockers and antihypertensive medications [see Drug Interactions ( 7.1) and Clinical Pharmacology ( 12.2)].
Caution is advised when PDE5 inhibitors are coadministered with alpha-blockers. PDE5 inhibitors, including tadalafil, and alpha-adrenergic blocking agents are both vasodilators with blood-pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly [see Drug Interactions ( 7.1) and Clinical Pharmacology ( 12.2)], which may lead to symptomatic hypotension (e.g., fainting). Consideration should be given to the following:
ED
• Patients should be stable on alpha-blocker therapy prior to initiating a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors.
• In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest recommended dose.
• In those patients already taking an optimized dose of PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor.
• Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other antihypertensive drugs. [see Dosage and Administration ( 2.7) and Drug Interactions ( 7.1)].
BPH
• The efficacy of the coadministration of an alpha-blocker and tadalafil for the treatment of BPH has not been adequately studied, and due to the potential vasodilatory effects of combined use resulting in blood pressure lowering, the combination of tadalafil and alpha-blockers is not recommended for the treatment of BPH. [see Dosage and Administration ( 2.7), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.2.)].
• Patients on alpha-blocker therapy for BPH should discontinue their alpha-blocker at least one day prior to starting tadalafil for once daily use for the treatment of BPH.
5.7 Renal Impairment
Tadalafil for Use as Needed
Tadalafil should be limited to 5 mg not more than once in every 72 hours in patients with creatinine clearance less than 30 mL/min or end-stage renal disease on hemodialysis. The starting dose of tadalafil in patients with creatinine clearance 30 to 50 mL/min should be 5 mg not more than once per day, and the maximum dose should be limited to 10 mg not more than once in every 48 hours [see Use in Specific Populations ( 8.7)].
Tadalafil for Once Daily Use
ED
Due to increased tadalafil exposure (AUC), limited clinical experience, and the lack of ability to influence clearance by dialysis, tadalafil for once daily use is not recommended in patients with creatinine clearance less than 30 mL/min [see Use in Specific Populations ( 8.7)].
BPH and ED/BPH
Due to increased tadalafil exposure (AUC), limited clinical experience, and the lack of ability to influence clearance by dialysis, tadalafil for once daily use is not recommended in patients with creatinine clearance less than 30 mL/min. In patients with creatinine clearance 30 to 50 mL/min, start dosing at 2.5 mg once daily, and increase the dose to 5 mg once daily based upon individual response [see Dosage and Administration ( 2.6), Use in Specific Populations ( 8.7), and Clinical Pharmacology ( 12.3)].
5.8 Hepatic Impairment
Tadalafil for Use as Needed
In patients with mild or moderate hepatic impairment, the dose of tadalafil should not exceed 10 mg. Because of insufficient information in patients with severe hepatic impairment, use of tadalafil in this group is not recommended [see Use in Specific Populations ( 8.6)].
Tadalafil for Once Daily Use
Tadalafil for once daily use has not been extensively evaluated in patients with mild or moderate hepatic impairment. Therefore, caution is advised if tadalafil tablets for once daily use is prescribed to these patients. Because of insufficient information in patients with severe hepatic impairment, use of tadalafil in this group is not recommended [see Use in Specific Populations ( 8.6)].5.9 Alcohol
Patients should be made aware that both alcohol and tadalafil, a PDE5 inhibitor, act as mild vasodilators. When mild vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., 5 units or greater) in combination with tadalafil can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Clinical Pharmacology ( 12.2)].
5.10 Concomitant Use of Potent Inhibitors of Cytochrome P450 3A4 (CYP3A4)
Tadalafil is metabolized predominantly by CYP3A4 in the liver. The dose of tadalafil for use as needed should be limited to 10 mg no more than once every 72 hours in patients taking potent inhibitors of CYP3A4 such as ritonavir, ketoconazole, and itraconazole [see Drug Interactions ( 7.2)]. In patients taking potent inhibitors of CYP3A4 and tadalafil for once daily use, the maximum recommended dose is 2.5 mg [see Dosage and Administration ( 2.7)].
5.11 Combination With Other PDE5 Inhibitors or Erectile Dysfunction Therapies
The safety and efficacy of combinations of tadalafil and other PDE5 inhibitors or treatments for erectile dysfunction have not been studied. Inform patients not to take tadalafil with other PDE5 inhibitors, including ADCIRCA.
5.12 Effects on Bleeding
Studies in vitrohave demonstrated that tadalafil is a selective inhibitor of PDE5. PDE5 is found in platelets. When administered in combination with aspirin, tadalafil 20 mg did not prolong bleeding time, relative to aspirin alone. Tadalafil has not been administered to patients with bleeding disorders or significant active peptic ulceration. Although tadalafil has not been shown to increase bleeding times in healthy subjects, use in patients with bleeding disorders or significant active peptic ulceration should be based upon a careful risk-benefit assessment and caution.
5.13 Counseling Patients About Sexually Transmitted Diseases
The use of tadalafil offers no protection against sexually transmitted diseases. Counseling patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV) should be considered.
Close5.14 Consideration of Other Urological Conditions Prior to Initiating Treatment for BPH
Prior to initiating treatment with tadalafil for BPH, consideration should be given to other urological conditions that may cause similar symptoms. In addition, prostate cancer and BPH may coexist.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Tadalafil was administered to over 9,000 men during clinical trials worldwide. In trials of tadalafil for once daily use, a total of 1,434, 905, and 115 were treated for at least 6 months, 1 year, and 2 years, respectively. For tadalafil for use as needed, over 1,300 and 1,000 subjects were treated for at least 6 months and 1 year, respectively.
Tadalafil for Use as Needed for ED
In eight primary placebo-controlled clinical studies of 12 weeks duration, mean age was 59 years (range 22 to 88) and the discontinuation rate due to adverse events in patients treated with tadalafil 10 or 20 mg was 3.1%, compared to 1.4% in placebo treated patients.
When taken as recommended in the placebo-controlled clinical trials, the following adverse reactions were reported ( seeTable 1) for tadalafil for use as needed:
Table 1: Treatment-Emergent Adverse Reactions Reported by ≥2% of Patients Treated with Tadalafil (10 or 20 mg) and More Frequent on Drug than Placebo in the Eight Primary Placebo-Controlled Clinical Studies (Including a Study in Patients with Diabetes) for Tadalafil for Use as Needed for ED
aThe term flushing includes: facial flushing and flushing Adverse Reaction
Placebo (N=476)
Tadalafil 5 mg (N=151)
Tadalafil 10 mg (N=394)
Tadalafil 20 mg (N=635)Headache
5%
11%
11%
15%Dyspepsia
1%
4%
8%
10%Back pain
3%
3%
5%
6%Myalgia
1%
1%
4%
3%Nasal congestion
1%
2%
3%
3%Flushing a
1%
2%
3%
3%P ain in limb
1%
1%
3%
3%
Tadalafil for Once Daily Use for ED
In three placebo-controlled clinical trials of 12 or 24 weeks duration, mean age was 58 years (range 21 to 82) and the discontinuation rate due to adverse events in patients treated with tadalafil was 4.1%, compared to 2.8% in placebo-treated patients.
Table 2: Treatment-Emergent Adverse Reactions Reported by ≥2% of Patients Treated with Tadalafil for Once Daily Use (2.5 or 5 mg) and More Frequent on Drug than Placebo in the Three Primary Placebo-Controlled Phase 3 Studies of 12 weeks Treatment Duration (Including a Study in Patients with Diabetes) for Tadalafil for Once Daily Use for ED
Adverse Reaction
Placebo
(N=248)
Tadalafil 2.5 mg
(N=196)
Tadalafil 5 mg
(N=304)
Headache
5%
3%
6%
Dyspepsia
2%
4%
5%
Nasopharyngitis
4%
4%
3%
Back pain
1%
3%
3%
Upper respiratory tract infection
1%
3%
3%
Flushing
1%
1%
3%
Myalgia
1%
2%
2%
Cough
0%
4%
2%
Diarrhea
0%
1%
2%
Nasal congestion
0%
2%
2%
Pain in extremity
0%
1%
2%
Urinary tract infection
0%
2%
0%
Gastroesophageal reflux disease
0%
2%
1%
Abdominal pain
0%
2%
1%
The following adverse reactions were reported ( seeTable 3) over 24 weeks treatment duration in one placebo-controlled clinical study:
Table 3: Treatment-Emergent Adverse Reactions Reported by ≥2% of Patients Treated with Tadalafil for Once Daily Use (2.5 or 5 mg) and More Frequent on Drug than Placebo in One Placebo-Controlled Clinical Study of 24 Weeks Treatment Duration for Tadalafil for Once Daily Use for ED
Adverse Reaction
Placebo
(N=94)
Tadalafil 2.5 mg
(N=96)
Tadalafil 5 mg
(N=97)
Nasopharyngitis
5%
6%
6%
Gastroenteritis
2%
3%
5%
Back pain
3%
5%
2%
Upper respiratory tract infection
0%
3%
4%
Dyspepsia
1%
4%
1%
Gastroesophageal reflux disease
0%
3%
2%
Myalgia
2%
4%
1%
Hypertension
0%
1%
3%
Nasal congestion
0%
0%
4%
Tadalafil for Once Daily Use for BPH and for ED and BPHIn three placebo-controlled clinical trials of 12 weeks duration, two in patients with BPH and one in patients with ED and BPH, the mean age was 63 years (range 44 to 93) and the discontinuation rate due to adverse events in patients treated with tadalafil was 3.6% compared to 1.6% in placebo-treated patients. Adverse reactions leading to discontinuation reported by at least 2 patients treated with tadalafil included headache, upper abdominal pain, and myalgia. The following adverse reactions were reported ( seeTable 4).
Table 4: Treatment-Emergent Adverse Reactions Reported by ≥1% of Patients Treated with Tadalafil for Once Daily Use (5 mg) and More Frequent on Drug than Placebo in Three Placebo-Controlled Clinical Studies of 12 Weeks Treatment Duration, including Two Studies for Tadalafil for Once Daily Use for BPH and One Study for ED and BPH
Adverse Reaction
Placebo
(N=576)
Tadalafil 5 mg
(N=581)
Headache
2.3%
4.1%
Dyspepsia
0.2%
2.4%
Back pain
1.4%
2.4%
Nasopharyngitis
1.6%
2.1%
Diarrhea
1%
1.4%
Pain in extremity
0%
1.4%
Myalgia
0.3%
1.2%
Dizziness
0.5%
1%
Additional, less frequent adverse reactions (<1%) reported in the controlled clinical trials of tadalafil for BPH or ED and BPH included: gastroesophageal reflux disease, upper abdominal pain, nausea, vomiting, arthralgia, and muscle spasm.
Back pain or myalgia was reported at incidence rates described in Tables 1 through 4. In tadalafil clinical pharmacology trials, back pain or myalgia generally occurred 12 to 24 hours after dosing and typically resolved within 48 hours. The back pain/myalgia associated with tadalafil treatment was characterized by diffuse bilateral lower lumbar, gluteal, thigh, or thoracolumbar muscular discomfort and was exacerbated by recumbency. In general, pain was reported as mild or moderate in severity and resolved without medical treatment, but severe back pain was reported with a low frequency (<5% of all reports). When medical treatment was necessary, acetaminophen or non-steroidal anti-inflammatory drugs were generally effective; however, in a small percentage of subjects who required treatment, a mild narcotic (e.g., codeine) was used. Overall, approximately 0.5% of all subjects treated with tadalafil for on demand use discontinued treatment as a consequence of back pain/myalgia. In the 1-year open label extension study, back pain and myalgia were reported in 5.5% and 1.3% of patients, respectively. Diagnostic testing, including measures for inflammation, muscle injury, or renal damage revealed no evidence of medically significant underlying pathology. Incidence rates for tadalafil for once daily use for ED, BPH and BPH/ED are described in Tables 2, 3 and 4. In studies of tadalafil for once daily use, adverse reactions of back pain and myalgia were generally mild or moderate with a discontinuation rate of <1% across all indications.
Across placebo-controlled studies with tadalafil for use as needed for ED, diarrhea was reported more frequently in patients 65 years of age and older who were treated with tadalafil (2.5% of patients) [see Use in Specific Populations ( 8.5)].
Across all studies with any tadalafil dose, reports of changes in color vision were rare (<0.1% of patients).
The following section identifies additional, less frequent events (<2%) reported in controlled clinical trials of tadalafil for once daily use or use as needed. A causal relationship of these events to tadalafil is uncertain. Excluded from this list are those events that were minor, those with no plausible relation to drug use, and reports too imprecise to be meaningful:
Body as a Whole— asthenia, face edema, fatigue, pain, peripheral edema
Cardiovascular— angina pectoris, chest pain, hypotension, myocardial infarction, postural hypotension, palpitations, syncope, tachycardia
Digestive— abnormal liver function tests, dry mouth, dysphagia, esophagitis, gastritis, GGTP increased, loose stools, nausea, upper abdominal pain, vomiting, gastroesophageal reflux disease, hemorrhoidal hemorrhage, rectal hemorrhage
Musculoskeletal— arthralgia, neck pain
Nervous— dizziness, hypesthesia, insomnia, paresthesia, somnolence, vertigo
Renal and Urinary— renal impairment
Respiratory— dyspnea, epistaxis, pharyngitis
Skin and Appendages— pruritus, rash, sweating
Ophthalmologic— blurred vision, changes in color vision, conjunctivitis (including conjunctival hyperemia), eye pain, lacrimation increase, swelling of eyelids
Otologic— sudden decrease or loss of hearing, tinnitus
Urogenital— erection increased, spontaneous penile erection
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of tadalafil.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion either due to their seriousness, reporting frequency, lack of clear alternative causation, or a combination of these factors.
Cardiovascular and Cerebrovascular— Serious cardiovascular events, including myocardial infarction, sudden cardiac death, stroke, chest pain, palpitations, and tachycardia, have been reported postmarketing in temporal association with the use of tadalafil. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of tadalafil without sexual activity. Others were reported to have occurred hours to days after the use of tadalafil and sexual activity. It is not possible to determine whether these events are related directly to tadalafil, to sexual activity, to the patient’s underlying cardiovascular disease, to a combination of these factors, or to other factors [see Warnings and Precautions ( 5.1)].
Body as a Whole— hypersensitivity reactions including urticaria, Stevens-Johnson syndrome, and exfoliative dermatitis
Nervous — migraine, seizure and seizure recurrence, transient global amnesia
Ophthalmologic — visual field defect, retinal vein occlusion, retinal artery occlusion
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely postmarketing in temporal association with the use of PDE5 inhibitors, including tadalafil. Most, but not all, of these patients had underlying anatomic or vascular risk factors for development of NAION, including but not necessarily limited to: low cup to disc ratio ("crowded disc"), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia, and smoking [see Warnings and Precautions ( 5.4)].
Otologic — Cases of sudden decrease or loss of hearing have been reported postmarketing in temporal association with the use of PDE5 inhibitors, including tadalafil. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of tadalafil, to the patient's underlying risk factors for hearing loss, a combination of these factors, or to other factors [see Warnings and Precautions ( 5.5)].
Urogenital— priapism [see Warnings and Precautions ( 5.3)].
-
7 DRUG INTERACTIONS7.1 Potential for Pharmacodynamic Interactions with Tadalafil - Nitrates— Administration of tadalafil to patients who are using any form of organic nitrate, is contraindicated. In ...
7.1 Potential for Pharmacodynamic Interactions with Tadalafil
Nitrates— Administration of tadalafil to patients who are using any form of organic nitrate, is contraindicated. In clinical pharmacology studies, tadalafil was shown to potentiate the hypotensive effect of nitrates. In a patient who has taken tadalafil, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 48 hours should elapse after the last dose of tadalafil before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring [see Dosage and Administration ( 2.7), Contraindications ( 4.1), and Clinical Pharmacology ( 12.2)].
Alpha-Blockers— Caution is advised when PDE5 inhibitors are coadministered with alpha-blockers. PDE5 inhibitors, including tadalafil, and alpha-adrenergic blocking agents are both vasodilators with blood-pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. Clinical pharmacology studies have been conducted with coadministration of tadalafil with doxazosin, tamsulosin or alfuzosin [see Dosage and Administration ( 2.7), Warnings and Precautions ( 5.6), and Clinical Pharmacology ( 12.2)].
Antihypertensives— PDE5 inhibitors, including tadalafil, are mild systemic vasodilators. Clinical pharmacology studies were conducted to assess the effect of tadalafil on the potentiation of the blood-pressure-lowering effects of selected antihypertensive medications (amlodipine, angiotensin II receptor blockers, bendrofluazide, enalapril, and metoprolol). Small reductions in blood pressure occurred following coadministration of tadalafil with these agents compared with placebo [see Warnings and Precautions ( 5.6) and Clinical Pharmacology ( 12.2)].
Alcohol— Both alcohol and tadalafil, a PDE5 inhibitor, act as mild vasodilators. When mild vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Substantial consumption of alcohol (e.g., 5 units or greater) in combination with tadalafil can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache. Tadalafil did not affect alcohol plasma concentrations and alcohol did not affect tadalafil plasma concentrations [see Warnings and Precautions ( 5.9) and Clinical Pharmacology ( 12.2)].7.2 Potential for Other Drugs to Affect Tadalafil
[See Dosage and Administration ( 2.7) and Warnings and Precautions ( 5.10)].
Antacids— Simultaneous administration of an antacid (magnesium hydroxide/aluminum hydroxide) and tadalafil reduced the apparent rate of absorption of tadalafil without altering exposure (AUC) to tadalafil.
H 2Antagonists (e.g. Nizatidine) — An increase in gastric pH resulting from administration of nizatidine had no significant effect on pharmacokinetics.
Cytochrome P450 Inhibitors— Tadalafil is a substrate of and predominantly metabolized by CYP3A4. Studies have shown that drugs that inhibit CYP3A4 can increase tadalafil exposure.
CYP3A4 (e.g., Ketoconazole)— Ketoconazole (400 mg daily), a selective and potent inhibitor of CYP3A4, increased tadalafil 20 mg single-dose exposure (AUC) by 312% and C maxby 22%, relative to the values for tadalafil 20 mg alone. Ketoconazole (200 mg daily) increased tadalafil 10 mg single-dose exposure (AUC) by 107% and C maxby 15%, relative to the values for tadalafil 10 mg alone [see Dosage and Administration ( 2.7)].
Although specific interactions have not been studied, other CYP3A4 inhibitors, such as erythromycin, itraconazole, and grapefruit juice, would likely increase tadalafil exposure.
HIV Protease inhibitor— Ritonavir (500 mg or 600 mg twice daily at steady state), an inhibitor of CYP3A4, CYP2C9, CYP2C19, and CYP2D6, increased tadalafil 20 mg single-dose exposure (AUC) by 32% with a 30% reduction in C max, relative to the values for tadalafil 20 mg alone. Ritonavir (200 mg twice daily), increased tadalafil 20 mg single-dose exposure (AUC) by 124% with no change in C max, relative to the values for tadalafil 20 mg alone. Although specific interactions have not been studied, other HIV protease inhibitors would likely increase tadalafil exposure [see Dosage and Administration ( 2.7)].
Cytochrome P450 Inducers— Studies have shown that drugs that induce CYP3A4 can decrease tadalafil exposure.
CYP3A4 (e.g., Rifampin)— Rifampin (600 mg daily), a CYP3A4 inducer, reduced tadalafil 10 mg single-dose exposure (AUC) by 88% and C maxby 46%, relative to the values for tadalafil 10 mg alone. Although specific interactions have not been studied, other CYP3A4 inducers, such as carbamazepine, phenytoin, and phenobarbital, would likely decrease tadalafil exposure. No dose adjustment is warranted. The reduced exposure of tadalafil with the coadministration of rifampin or other CYP3A4 inducers can be anticipated to decrease the efficacy of tadalafil for once daily use; the magnitude of decreased efficacy is unknown.Close7.3 Potential for Tadalafil to Affect Other Drugs
Aspirin— Tadalafil did not potentiate the increase in bleeding time caused by aspirin.
Cytochrome P450 Substrates— Tadalafil is not expected to cause clinically significant inhibition or induction of the clearance of drugs metabolized by cytochrome P450 (CYP) isoforms. Studies have shown that tadalafil does not inhibit or induce P450 isoforms CYP1A2, CYP3A4, CYP2C9, CYP2C19, CYP2D6, and CYP2E1.
CYP1A2 (e.g. Theophylline)— Tadalafil had no significant effect on the pharmacokinetics of theophylline. When tadalafil was administered to subjects taking theophylline, a small augmentation (3 beats per minute) of the increase in heart rate associated with theophylline was observed.
CYP2C9 (e.g. Warfarin)— Tadalafil had no significant effect on exposure (AUC) to S-warfarin or R-warfarin, nor did tadalafil affect changes in prothrombin time induced by warfarin.
CYP3A4 (e.g. Midazolam or Lovastatin)— Tadalafil had no significant effect on exposure (AUC) to midazolam or lovastatin.
P-glycoprotein (e.g. Digoxin)— Coadministration of tadalafil (40 mg once per day) for 10 days did not have a significant effect on the steady-state pharmacokinetics of digoxin (0.25 mg/day) in healthy subjects. -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary Tadalafil is not indicated for use in females. There are no data with the use of tadalafil in pregnant women to inform any ...
8.1 Pregnancy
Risk Summary
Tadalafil is not indicated for use in females.
There are no data with the use of tadalafil in pregnant women to inform any drug-associated risks for adverse developmental outcomes. In animal reproduction studies, no adverse developmental effects were observed with oral administration of tadalafil to pregnant rats or mice during organogenesis at exposures up to 11 times the maximum recommended human dose (MRHD) of 20 mg/day ( seeData).
Data
Animal Data
Animal reproduction studies showed no evidence of teratogenicity, embryotoxicity, or fetotoxicity when tadalafil was given orally to pregnant rats or mice at exposures up to 11 times the maximum recommended human dose (MRHD) of 20 mg/day during organogenesis. In a prenatal/postnatal developmental study in rats, postnatal pup survival decreased following maternal exposure to tadalafil doses greater than 10 times the MRHD based on AUC. Signs of maternal toxicity occurred at doses greater than 16 times the MRHD based on AUC. Surviving offspring had normal development and reproductive performance.
In another rat prenatal and postnatal development study at doses of 60, 200, and 1,000 mg/kg, a reduction in postnatal survival of pups was observed. The no observed effect level (NOEL) for maternal toxicity was 200 mg/kg/day and for developmental toxicity was 30 mg/kg/day. This gives approximately 16 and 10 fold exposure multiples, respectively, of the human AUC for the MRHD of 20 mg.
Tadalafil and/or its metabolites cross the placenta, resulting in fetal exposure in rats.8.2 Lactation
Risk Summary
Tadalafil is not indicated for use in females.
There is no information on the presence of tadalafil and/or metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Tadalafil and/or its metabolites are present in the milk of lactating rats at concentrations approximately 2.4-fold greater than found in the plasma.
8.3 Females and Males of Reproductive Potential
Infertility
Based on the data from 3 studies in adult males, tadalafil decreased sperm concentrations in the study of 10 mg tadalafil for 6 months and the study of 20 mg tadalafil for 9 months. This effect was not seen in the study of 20 mg tadalafil taken for 6 months. There was no adverse effect of tadalafil 10 mg or 20 mg on mean concentrations of testosterone, luteinizing hormone or follicle stimulating hormone. The clinical significance of the decreased sperm concentrations in the two studies is unknown. There have been no studies evaluating the effect of tadalafil on fertility in men [see Clinical Pharmacology ( 12.2)].
Based on studies in animals, a decrease in spermatogenesis was observed in dogs, but not in rats [see Nonclinical Toxicology ( 13.1)].
8.4 Pediatric Use
Tadalafil is not indicated for use in pediatric patients. Safety and efficacy in patients below the age of 18 years have not been established.
Juvenile Animal Study
No adverse effects were observed in a study in which tadalafil was administered orally at doses of 60, 200, and 1,000 mg/kg/day to juvenile rats on postnatal days 14 to 90. The highest plasma tadalafil exposures (AUC) achieved were approximately 10-fold that observed at the MRHD.
Additional information describing a clinical study in which efficacy was not demonstrated is approved for Eli Lilly and Company's CIALIS (tadalafil) tablets. However, due to Eli Lilly and Company's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
Of the total number of subjects in ED clinical studies of tadalafil, approximately 19 percent were 65 and over, while approximately 2 percent were 75 and over. Of the total number of subjects in BPH clinical studies of tadalafil (including the ED/BPH study), approximately 40 percent were over 65, while approximately 10 percent were 75 and over. In these clinical trials, no overall differences in efficacy or safety were observed between older (>65 and ≥75 years of age) and younger subjects (≤65 years of age). However, in placebo-controlled studies with tadalafil for use as needed for ED, diarrhea was reported more frequently in patients 65 years of age and older who were treated with tadalafil (2.5% of patients) [see Adverse Reactions ( 6.1)]. No dose adjustment is warranted based on age alone. However, a greater sensitivity to medications in some older individuals should be considered [see Clinical Pharmacology ( 12.3)].
8.6 Hepatic Impairment
In clinical pharmacology studies, tadalafil exposure (AUC) in subjects with mild or moderate hepatic impairment (Child-Pugh Class A or B) was comparable to exposure in healthy subjects when a dose of 10 mg was administered. There are no available data for doses higher than 10 mg of tadalafil in patients with hepatic impairment. Insufficient data are available for subjects with severe hepatic impairment (Child-Pugh Class C) [see Dosage and Administration ( 2.6) and Warnings and Precautions ( 5.8)].
Close8.7 Renal Impairment
In clinical pharmacology studies using single-dose tadalafil (5 to 10 mg), tadalafil exposure (AUC) doubled in subjects with creatinine clearance 30 to 80 mL/min. In subjects with end-stage renal disease on hemodialysis, there was a two-fold increase in C maxand 2.7- to 4.8-fold increase in AUC following single-dose administration of 10 or 20 mg tadalafil. Exposure to total methylcatechol (unconjugated plus glucuronide) was 2- to 4-fold higher in subjects with renal impairment, compared to those with normal renal function. Hemodialysis (performed between 24 and 30 hours post-dose) contributed negligibly to tadalafil or metabolite elimination. In a clinical pharmacology study (N=28) at a dose of 10 mg, back pain was reported as a limiting adverse event in male patients with creatinine clearance 30 to 50 mL/min. At a dose of 5 mg, the incidence and severity of back pain was not significantly different than in the general population. In patients on hemodialysis taking 10 or 20 mg tadalafil, there were no reported cases of back pain [see Dosage and Administration ( 2.6) and Warnings and Precautions ( 5.7)].
-
10 OVERDOSAGESingle doses up to 500 mg have been given to healthy subjects, and multiple daily doses up to 100 mg have been given to patients. Adverse events were similar to those seen at lower doses ...
Single doses up to 500 mg have been given to healthy subjects, and multiple daily doses up to 100 mg have been given to patients. Adverse events were similar to those seen at lower doses. In cases of overdose, standard supportive measures should be adopted as required. Hemodialysis contributes negligibly to tadalafil elimination.
Close -
11 DESCRIPTIONTadalafil is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the molecular formula C - 22H - 19N - 3O ...
Tadalafil is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the molecular formula C 22H 19N 3O 4representing a molecular weight of 389.41. The structural formula is:

The chemical designation is pyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione,6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-(6R-12aR)-. Tadalafil USP is a white or almost white powder that is freely soluble in dimethyl sulfoxide, slightly soluble in methylene chloride and practically insoluble in water.
Tadalafil tablets, USP are available as round (2.5 mg and 5 mg) and capsule (10 mg and 20 mg) shaped tablets for oral administration. Each tablet contains 2.5 mg, 5 mg, 10 mg, or 20 mg of tadalafil USP and the following inactive ingredients: colloidal silicon dioxide, copovidone, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyoxyl 40 hydrogenated castor oil, talc, titanium dioxide and triacetin. In addition, 2.5 mg contains FD&C blue #2/indigo carmine aluminum lake.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosal ...
12.1 Mechanism of Action
Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle. This response is mediated by the release of nitric oxide (NO) from nerve terminals and endothelial cells, which stimulates the synthesis of cGMP in smooth muscle cells. Cyclic GMP causes smooth muscle relaxation and increased blood flow into the corpus cavernosum. The inhibition of phosphodiesterase type 5 (PDE5) enhances erectile function by increasing the amount of cGMP. Tadalafil inhibits PDE5. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 by tadalafil has no effect in the absence of sexual stimulation.
The effect of PDE5 inhibition on cGMP concentration in the corpus cavernosum and pulmonary arteries is also observed in the smooth muscle of the prostate, the bladder and their vascular supply. The mechanism for reducing BPH symptoms has not been established.
Studies in vitrohave demonstrated that tadalafil is a selective inhibitor of PDE5. PDE5 is found in the smooth muscle of the corpus cavernosum, prostate, and bladder as well as in vascular and visceral smooth muscle, skeletal muscle, urethra, platelets, kidney, lung, cerebellum, heart, liver, testis, seminal vesicle, and pancreas.
In vitrostudies have shown that the effect of tadalafil is more potent on PDE5 than on other phosphodiesterases. These studies have shown that tadalafil is >10,000-fold more potent for PDE5 than for PDE1, PDE2, PDE4, and PDE7 enzymes, which are found in the heart, brain, blood vessels, liver, leukocytes, skeletal muscle, and other organs. Tadalafil is >10,000-fold more potent for PDE5 than for PDE3, an enzyme found in the heart and blood vessels. Additionally, tadalafil is 700-fold more potent for PDE5 than for PDE6, which is found in the retina and is responsible for phototransduction. Tadalafil is >9,000-fold more potent for PDE5 than for PDE8, PDE9, and PDE10. Tadalafil is 14-fold more potent for PDE5 than for PDE11A1 and 40-fold more potent for PDE5 than for PDE11A4, two of the four known forms of PDE11. PDE11 is an enzyme found in human prostate, testes, skeletal muscle and in other tissues (e.g., adrenal cortex). In vitro, tadalafil inhibits human recombinant PDE11A1 and, to a lesser degree, PDE11A4 activities at concentrations within the therapeutic range. The physiological role and clinical consequence of PDE11 inhibition in humans have not been defined.
12.2 Pharmacodynamics
Effects on Blood Pressure
Tadalafil 20 mg administered to healthy male subjects produced no significant difference compared to placebo in supine systolic and diastolic blood pressure (difference in the mean maximal decrease of 1.6/0.8 mm Hg, respectively) and in standing systolic and diastolic blood pressure (difference in the mean maximal decrease of 0.2/4.6 mm Hg, respectively). In addition, there was no significant effect on heart rate.
Effects on Blood Pressure When Administered with Nitrates
In clinical pharmacology studies, tadalafil (5 to 20 mg) was shown to potentiate the hypotensive effect of nitrates. Therefore, the use of tadalafil in patients taking any form of nitrates is contraindicated [see Contraindications ( 4.1)].
A study was conducted to assess the degree of interaction between nitroglycerin and tadalafil, should nitroglycerin be required in an emergency situation after tadalafil was taken. This was a double-blind, placebo-controlled, crossover study in 150 male subjects at least 40 years of age (including subjects with diabetes mellitus and/or controlled hypertension) and receiving daily doses of tadalafil 20 mg or matching placebo for 7 days. Subjects were administered a single dose of 0.4 mg sublingual nitroglycerin (NTG) at pre-specified timepoints, following their last dose of tadalafil (2, 4, 8, 24, 48, 72, and 96 hours after tadalafil). The objective of the study was to determine when, after tadalafil dosing, no apparent blood pressure interaction was observed. In this study, a significant interaction between tadalafil and NTG was observed at each timepoint up to and including 24 hours. At 48 hours, by most hemodynamic measures, the interaction between tadalafil and NTG was not observed, although a few more tadalafil subjects compared to placebo experienced greater blood-pressure lowering at this timepoint. After 48 hours, the interaction was not detectable ( seeFigure 1).

Figure 1: Mean Maximal Change in Blood Pressure (Tadalafil Minus Placebo, Point Estimate with 90% CI) in Response to Sublingual Nitroglycerin at 2 (Supine Only), 4, 8, 24, 48, 72, and 96 Hours after the Last Dose of Tadalafil 20 mg or Placebo
Therefore, tadalafil administration with nitrates is contraindicated. In a patient who has taken tadalafil, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 48 hours should elapse after the last dose of tadalafil before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications ( 4.1)].
Effect on Blood Pressure When Administered With Alpha-Blockers
Six randomized, double-blinded, crossover clinical pharmacology studies were conducted to investigate the potential interaction of tadalafil with alpha-blocker agents in healthy male subjects [see Dosage and Administration ( 2.7) and Warnings and Precautions ( 5.6)] . In four studies, a single oral dose of tadalafil was administered to healthy male subjects taking daily (at least 7 days duration) an oral alpha-blocker. In two studies, a daily oral alpha-blocker (at least 7 days duration) was administered to healthy male subjects taking repeated daily doses of tadalafil.
Doxazosin— Three clinical pharmacology studies were conducted with tadalafil and doxazosin, an alpha[1]-adrenergic blocker.
In the first doxazosin study, a single oral dose of tadalafil 20 mg or placebo was administered in a 2-period, crossover design to healthy subjects taking oral doxazosin 8 mg daily (N=18 subjects). Doxazosin was administered at the same time as tadalafil or placebo after a minimum of seven days of doxazosin dosing ( seeTable 5 and Figure 2).
Table 5: Doxazosin (8 mg/day) Study 1: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg)
Tadalafil 20 mg
Supine
3.6 (-1.5, 8.8)
Standing
9.8 (4.1, 15.5)
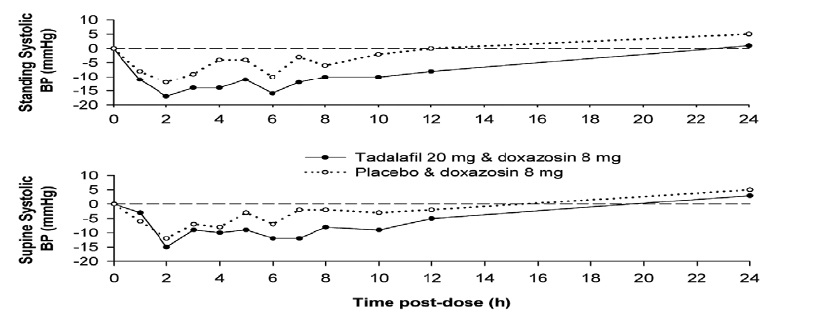
Figure 2: Doxazosin Study 1: Mean Change from Baseline in Systolic Blood Pressure
Blood pressure was measured manually at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours after tadalafil or placebo administration. Outliers were defined as subjects with a standing systolic blood pressure of <85 mm Hg or a decrease from baseline in standing systolic blood pressure of >30 mm Hg at one or more time points. There were nine and three outliers following administration of tadalafil 20 mg and placebo, respectively. Five and two subjects were outliers due to a decrease from baseline in standing systolic BP of >30 mm Hg, while five and one subject were outliers due to standing systolic BP <85 mm Hg following tadalafil and placebo, respectively. Severe adverse events potentially related to blood-pressure effects were assessed. No such events were reported following placebo. Two such events were reported following administration of tadalafil. Vertigo was reported in one subject that began 7 hours after dosing and lasted about 5 days. This subject previously experienced a mild episode of vertigo on doxazosin and placebo. Dizziness was reported in another subject that began 25 minutes after dosing and lasted 1 day. No syncope was reported.
In the second doxazosin study, a single oral dose of tadalafil 20 mg was administered to healthy subjects taking oral doxazosin, either 4 mg or 8 mg daily. The study (N=72 subjects) was conducted in three parts, each a 3-period crossover.
In part A (N=24), subjects were titrated to doxazosin 4 mg administered daily at 8 a.m. Tadalafil was administered at either 8 a.m., 4 p.m., or 8 p.m. There was no placebo control.
In part B (N=24), subjects were titrated to doxazosin 4 mg administered daily at 8 p.m. Tadalafil was administered at either 8 a.m., 4 p.m., or 8 p.m. There was no placebo control.
In part C (N=24), subjects were titrated to doxazosin 8 mg administered daily at 8 a.m. In this part, tadalafil or placebo were administered at either 8 a.m. or 8 p.m.
The placebo-subtracted mean maximal decreases in systolic blood pressure over a 12-hour period after dosing in the placebo-controlled portion of the study (part C) are shown in Table 6 and Figure 3.
Table 6: Doxazosin (8 mg/day) Study 2 (Part C): Mean Maximal Decrease in Systolic Blood Pressure
Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg)
Tadalafil 20 mg at 8 a.m.
Tadalafil 20 mg at 8 p.m.
Ambulatory Blood-Pressure Monitoring (ABPM)
7
8
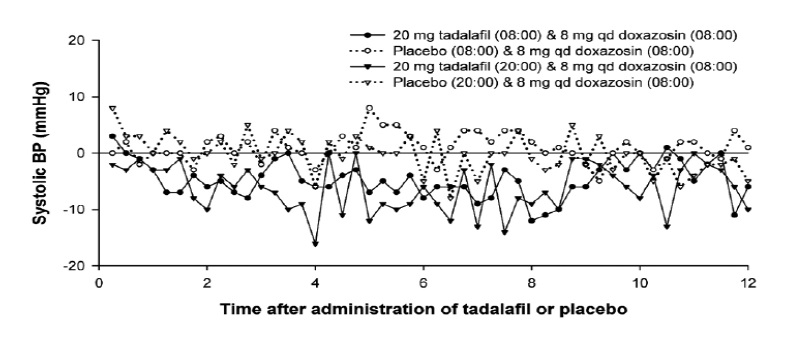
Figure 3: Doxazosin Study 2 (Part C): Mean Change from Time-Matched Baseline in Systolic Blood Pressure
Blood pressure was measured by ABPM every 15 to 30 minutes for up to 36 hours after tadalafil or placebo. Subjects were categorized as outliers if one or more systolic blood pressure readings of <85 mm Hg were recorded or one or more decreases in systolic blood pressure of >30 mm Hg from a time-matched baseline occurred during the analysis interval.
Of the 24 subjects in part C, 16 subjects were categorized as outliers following administration of tadalafil and 6 subjects were categorized as outliers following placebo during the 24-hour period after 8 a.m. dosing of tadalafil or placebo. Of these, 5 and 2 were outliers due to systolic BP <85 mm Hg, while 15 and 4 were outliers due to a decrease from baseline in systolic BP of >30 mm Hg following tadalafil and placebo, respectively.
During the 24-hour period after 8 p.m. dosing, 17 subjects were categorized as outliers following administration of tadalafil and 7 subjects following placebo. Of these, 10 and 2 subjects were outliers due to systolic BP <85 mm Hg, while 15 and 5 subjects were outliers due to a decrease from baseline in systolic BP of >30 mm Hg, following tadalafil and placebo, respectively.
Some additional subjects in both the tadalafil and placebo groups were categorized as outliers in the period beyond 24 hours.
Severe adverse events potentially related to blood-pressure effects were assessed. In the study (N=72 subjects), 2 such events were reported following administration of tadalafil (symptomatic hypotension in one subject that began 10 hours after dosing and lasted approximately 1 hour, and dizziness in another subject that began 11 hours after dosing and lasted 2 minutes). No such events were reported following placebo. In the period prior to tadalafil dosing, one severe event (dizziness) was reported in a subject during the doxazosin run-in phase.
In the third doxazosin study, healthy subjects (N=45 treated; 37 completed) received 28 days of once per day dosing of tadalafil 5 mg or placebo in a two-period crossover design. After 7 days, doxazosin was initiated at 1 mg and titrated up to 4 mg daily over the last 21 days of each period (7 days on 1 mg; 7 days of 2 mg; 7 days of 4 mg doxazosin). The results are shown in Table 7.
Table 7: Doxazosin Study 3: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
Placebo-subtracted mean maximal decrease in systolic blood pressure
Tadalafil 5 mg
Day 1 of 4 mg Doxazosin
Supine2.4 (-0.4, 5.2)
Standing-0.5 (-4, 3.1)
Day 7 of 4 mg Doxazosin
Supine2.8 (-0.1, 5.7)
Standing1.1 (-2.9, 5)
Blood pressure was measured manually pre-dose at two time points (-30 and -15 minutes) and then at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12 and 24 hours post dose on the first day of each doxazosin dose, (1 mg, 2 mg, 4 mg), as well as on the seventh day of 4 mg doxazosin administration.
Following the first dose of doxazosin 1 mg, there were no outliers on tadalafil 5 mg and one outlier on placebo due to a decrease from baseline in standing systolic BP of >30 mm Hg.
There were 2 outliers on tadalafil 5 mg and none on placebo following the first dose of doxazosin 2 mg due to a decrease from baseline in standing systolic BP of >30 mm Hg.
There were no outliers on tadalafil 5 mg and two on placebo following the first dose of doxazosin 4 mg due to a decrease from baseline in standing systolic BP of >30 mm Hg. There was one outlier on tadalafil 5 mg and three on placebo following the first dose of doxazosin 4 mg due to standing systolic BP <85 mm Hg. Following the seventh day of doxazosin 4 mg, there were no outliers on tadalafil 5 mg, one subject on placebo had a decrease >30 mm Hg in standing systolic blood pressure, and one subject on placebo had standing systolic blood pressure <85 mm Hg. All adverse events potentially related to blood pressure effects were rated as mild or moderate. There were two episodes of syncope in this study, one subject following a dose of tadalafil 5 mg alone, and another subject following coadministration of tadalafil 5 mg and doxazosin 4 mg.
Tamsulosin— In the first tamsulosin study, a single oral dose of tadalafil 10, 20 mg, or placebo was administered in a 3 period, crossover design to healthy subjects taking 0.4 mg once per day tamsulosin, a selective alpha[1A]-adrenergic blocker (N=18 subjects). Tadalafil or placebo was administered 2 hours after tamsulosin following a minimum of seven days of tamsulosin dosing.
Table 8: Tamsulosin (0.4 mg/day) Study 1: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg)
Tadalafil 10 mg
Tadalafil 20 mg
Supine
3.2 (-2.3, 8.6)
3.2 (-2.3, 8.7)
Satnding
1.7 (-4.7, 8.1)
2.3 (-4.1, 8.7)
Blood pressure was measured manually at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours after tadalafil or placebo dosing. There were 2, 2, and 1 outliers (subjects with a decrease from baseline in standing systolic blood pressure of >30 mm Hg at one or more time points) following administration of tadalafil 10 mg, 20 mg, and placebo, respectively. There were no subjects with a standing systolic blood pressure <85 mm Hg. No severe adverse events potentially related to blood-pressure effects were reported. No syncope was reported.
In the second tamsulosin study, healthy subjects (N=39 treated; and 35 completed) received 14 days of once per day dosing of tadalafil 5 mg or placebo in a two-period crossover design. Daily dosing of tamsulosin 0.4 mg was added for the last seven days of each period.
Table 9: Tamsulosin Study 2: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
Placebo-subtracted mean maximal decrease in systolic blood pressure
Tadalafil 5 mg
Day 1 of 0.4 mg Tamsulosin
Supine-0.1 (-2.2, 1.9)
Standing
0.9 (-1.4, 3.2)
Day 7 of 0.4 mg Tamsulosin
Supine
1.2 (-1.2, 3.6)
Standing
1.2 (-1, 3.5)
Blood pressure was measured manually pre-dose at two time points (-30 and -15 minutes) and then at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours post dose on the first, sixth and seventh days of tamsulosin administration. There were no outliers (subjects with a decrease from baseline in standing systolic blood pressure of >30 mm Hg at one or more time points). One subject on placebo plus tamsulosin (Day 7) and one subject on tadalafil plus tamsulosin (Day 6) had standing systolic blood pressure <85 mm Hg. No severe adverse events potentially related to blood pressure were reported. No syncope was reported.
Alfuzosin — A single oral dose of tadalafil 20 mg or placebo was administered in a 2-period, crossover design to healthy subjects taking once-daily alfuzosin HCl 10 mg extended-release tablets, an alpha[1]-adrenergic blocker (N=17 completed subjects). Tadalafil or placebo was administered 4 hours after alfuzosin following a minimum of seven days of alfuzosin dosing.
Table 10: Alfuzosin (10 mg/day) Study: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg)
Tadalafil 20 mg
Supine2.2 (-0.9, -5.2)
Standing4.4 (-0.2, 8.9)
Blood pressure was measured manually at 1, 2, 3, 4, 6, 8, 10, 20, and 24 hours after tadalafil or placebo dosing. There was 1 outlier (subject with a standing systolic blood pressure <85 mm Hg) following administration of tadalafil 20 mg. There were no subjects with a decrease from baseline in standing systolic blood pressure of >30 mm Hg at one or more time points. No severe adverse events potentially related to blood pressure effects were reported. No syncope was reported.
Effects on Blood Pressure When Administered with Antihypertensives
Amlodipine— A study was conducted to assess the interaction of amlodipine (5 mg daily) and tadalafil 10 mg. There was no effect of tadalafil on amlodipine blood levels and no effect of amlodipine on tadalafil blood levels. The mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking amlodipine was 3/2 mm Hg, compared to placebo. In a similar study using tadalafil 20 mg, there were no clinically significant differences between tadalafil and placebo in subjects taking amlodipine.
Angiotensin II receptor blockers(with and without other antihypertensives) — A study was conducted to assess the interaction of angiotensin II receptor blockers and tadalafil 20 mg. Subjects in the study were taking any marketed angiotensin II receptor blocker, either alone, as a component of a combination product, or as part of a multiple antihypertensive regimen. Following dosing, ambulatory measurements of blood pressure revealed differences between tadalafil and placebo of 8/4 mm Hg in systolic/diastolic blood pressure.
Bendrofluazide— A study was conducted to assess the interaction of bendrofluazide (2.5 mg daily) and tadalafil 10 mg. Following dosing, the mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking bendrofluazide was 6/4 mm Hg, compared to placebo.
Enalapril— A study was conducted to assess the interaction of enalapril (10 to 20 mg daily) and tadalafil 10 mg. Following dosing, the mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking enalapril was 4/1 mm Hg, compared to placebo.
Metoprolol— A study was conducted to assess the interaction of sustained-release metoprolol (25 to 200 mg daily) and tadalafil 10 mg. Following dosing, the mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking metoprolol was 5/3 mm Hg, compared to placebo.
Effects on Blood Pressure When Administered with Alcohol
Alcohol and PDE5 inhibitors, including tadalafil, are mild systemic vasodilators. The interaction of tadalafil with alcohol was evaluated in 3 clinical pharmacology studies. In 2 of these, alcohol was administered at a dose of 0.7 g/kg, which is equivalent to approximately 6 ounces of 80-proof vodka in an 80-kg male, and tadalafil was administered at a dose of 10 mg in one study and 20 mg in another. In both these studies, all patients imbibed the entire alcohol dose within 10 minutes of starting. In one of these two studies, blood alcohol levels of 0.08% were confirmed. In these two studies, more patients had clinically significant decreases in blood pressure on the combination of tadalafil and alcohol as compared to alcohol alone. Some subjects reported postural dizziness, and orthostatic hypotension was observed in some subjects. When tadalafil 20 mg was administered with a lower dose of alcohol (0.6 g/kg, which is equivalent to approximately 4 ounces of 80-proof vodka, administered in less than 10 minutes), orthostatic hypotension was not observed, dizziness occurred with similar frequency to alcohol alone, and the hypotensive effects of alcohol were not potentiated.
Tadalafil did not affect alcohol plasma concentrations and alcohol did not affect tadalafil plasma concentrations.
Effects on Exercise Stress Testing
The effects of tadalafil on cardiac function, hemodynamics, and exercise tolerance were investigated in a single clinical pharmacology study. In this blinded crossover trial, 23 subjects with stable coronary artery disease and evidence of exercise-induced cardiac ischemia were enrolled. The primary endpoint was time to cardiac ischemia. The mean difference in total exercise time was 3 seconds (tadalafil 10 mg minus placebo), which represented no clinically meaningful difference. Further statistical analysis demonstrated that tadalafil was non-inferior to placebo with respect to time to ischemia. Of note, in this study, in some subjects who received tadalafil followed by sublingual nitroglycerin in the post-exercise period, clinically significant reductions in blood pressure were observed, consistent with the augmentation by tadalafil of the blood-pressure-lowering effects of nitrates.
Effects on Vision
Single oral doses of phosphodiesterase inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green), using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina. In a study to assess the effects of a single dose of tadalafil 40 mg on vision (N=59), no effects were observed on visual acuity, intraocular pressure, or pupilometry. Across all clinical studies with tadalafil, reports of changes in color vision were rare (<0.1% of patients).
Effects on Sperm Characteristics
Three studies were conducted in men to assess the potential effect on sperm characteristics of tadalafil 10 mg (one 6 month study) and 20 mg (one 6 month and one 9 month study) administered daily. There were no adverse effects on sperm morphology or sperm motility in any of the three studies. In the study of 10 mg tadalafil for 6 months and the study of 20 mg tadalafil for 9 months, results showed a decrease in mean sperm concentrations relative to placebo, although these differences were not clinically meaningful. This effect was not seen in the study of 20 mg tadalafil taken for 6 months. In addition there was no adverse effect on mean concentrations of reproductive hormones, testosterone, luteinizing hormone or follicle stimulating hormone with either 10 or 20 mg of tadalafil compared to placebo.
Effects on Cardiac Electrophysiology
The effect of a single 100 mg dose of tadalafil on the QT interval was evaluated at the time of peak tadalafil concentration in a randomized, double-blinded, placebo, and active (intravenous ibutilide) -controlled crossover study in 90 healthy males aged 18 to 53 years. The mean change in QTc (Fridericia QT correction) for tadalafil, relative to placebo, was 3.5 milliseconds (two-sided 90% CI=1.9, 5.1). The mean change in QTc (Individual QT correction) for tadalafil, relative to placebo, was 2.8 milliseconds (two-sided 90% CI=1.2, 4.4). A 100 mg dose of tadalafil (5 times the highest recommended dose) was chosen because this dose yields exposures covering those observed upon coadministration of tadalafil with potent CYP3A4 inhibitors or those observed in renal impairment. In this study, the mean increase in heart rate associated with a 100 mg dose of tadalafil compared to placebo was 3.1 beats per minute.
Close12.3 Pharmacokinetics
Over a dose range of 2.5 to 20 mg, tadalafil exposure (AUC) increases proportionally with dose in healthy subjects. Steady-state plasma concentrations are attained within 5 days of once per day dosing and exposure is approximately 1.6-fold greater than after a single dose. Mean tadalafil concentrations measured after the administration of a single oral dose of 20 mg and single and once daily multiple doses of 5 mg, from a separate study, ( seeFigure 4) to healthy male subjects are depicted in Figure 4.

Figure 4: Plasma tadalafil concentrations (mean ± SD) following a single 20 mg tadalafil dose and single and once daily multiple doses of 5 mg
Absorption— After single oral-dose administration, the maximum observed plasma concentration (C max) of tadalafil is achieved between 30 minutes and 6 hours (median time of 2 hours). Absolute bioavailability of tadalafil following oral dosing has not been determined.
The rate and extent of absorption of tadalafil are not influenced by food; thus tadalafil may be taken with or without food.
Distribution— The mean apparent volume of distribution following oral administration is approximately 63 L, indicating that tadalafil is distributed into tissues. At therapeutic concentrations, 94% of tadalafil in plasma is bound to proteins.
Less than 0.0005% of the administered dose appeared in the semen of healthy subjects.
Metabolism— Tadalafil is predominantly metabolized by CYP3A4 to a catechol metabolite. The catechol metabolite undergoes extensive methylation and glucuronidation to form the methylcatechol and methylcatechol glucuronide conjugate, respectively. The major circulating metabolite is the methylcatechol glucuronide. Methylcatechol concentrations are less than 10% of glucuronide concentrations. In vitrodata suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
Excretion— The mean oral clearance for tadalafil is 2.5 L/hr and the mean terminal half-life is 17.5 hours in healthy subjects. Tadalafil is excreted predominantly as metabolites, mainly in the feces (approximately 61% of the dose) and to a lesser extent in the urine (approximately 36% of the dose).
Geriatric— Healthy male elderly subjects (65 years or over) had a lower oral clearance of tadalafil, resulting in 25% higher exposure (AUC) with no effect on C maxrelative to that observed in healthy subjects 19 to 45 years of age. No dose adjustment is warranted based on age alone. However, greater sensitivity to medications in some older individuals should be considered [see Use in Specific Populations ( 8.5)].
Patients with Diabetes Mellitus— In male patients with diabetes mellitus after a 10 mg tadalafil dose, exposure (AUC) was reduced approximately 19% and C maxwas 5% lower than that observed in healthy subjects. No dose adjustment is warranted.
Patients with BPH— In patients with BPH following single and multiple-doses of 20 mg tadalafil, no statistically significant differences in exposure (AUC and C max) were observed between elderly (70 to 85 years) and younger (≤60 years of age) subjects. No dose adjustment is warranted.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis— Tadalafil was not carcinogenic to rats or mice when administered daily for 2 years at doses up to 400 ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis— Tadalafil was not carcinogenic to rats or mice when administered daily for 2 years at doses up to 400 mg/kg/day. Systemic drug exposures, as measured by AUC of unbound tadalafil, were approximately 10-fold for mice, and 14- and 26-fold for male and female rats, respectively, the exposures in human males given Maximum Recommended Human Dose (MRHD) of 20 mg.
Mutagenesis— Tadalafil was not mutagenic in the in vitrobacterial Ames assays or the forward mutation test in mouse lymphoma cells. Tadalafil was not clastogenic in the in vitrochromosomal aberration test in human lymphocytes or the in vivorat micronucleus assays.
Impairment of Fertility— There were no effects on fertility, reproductive performance or reproductive organ morphology in male or female rats given oral doses of tadalafil up to 400 mg/kg/day, a dose producing AUCs for unbound tadalafil of 14-fold for males or 26-fold for females the exposures observed in human males given the MRHD of 20 mg. In beagle dogs given tadalafil daily for 3 to 12 months, there was treatment-related non-reversible degeneration and atrophy of the seminiferous tubular epithelium in the testes in 20 to 100% of the dogs that resulted in a decrease in spermatogenesis in 40 to 75% of the dogs at doses of ≥10 mg/kg/day. Systemic exposure (based on AUC) at no-observed-adverse-effect-level (NOAEL) (10 mg/kg/day) for unbound tadalafil was similar to that expected in humans at the MRHD of 20 mg.
There were no treatment-related testicular findings in rats or mice treated with doses up to 400 mg/kg/day for 2 years.
Close13.2 Animal Pharmacology and/or Toxicology
Animal studies showed vascular inflammation in tadalafil-treated mice, rats, and dogs. In mice and rats, lymphoid necrosis and hemorrhage were seen in the spleen, thymus, and mesenteric lymph nodes at unbound tadalafil exposure of 2- to 33-fold above the human exposure (AUCs) at the MRHD of 20 mg. In dogs, an increased incidence of disseminated arteritis was observed in 1- and 6-month studies at unbound tadalafil exposure of 1- to 54-fold above the human exposure (AUC) at the MRHD of 20 mg. In a 12-month dog study, no disseminated arteritis was observed, but 2 dogs exhibited marked decreases in white blood cells (neutrophils) and moderate decreases in platelets with inflammatory signs at unbound tadalafil exposures of approximately 14- to 18-fold the human exposure at the MRHD of 20 mg. The abnormal blood-cell findings were reversible within 2 weeks after stopping treatment.
-
14 CLINICAL STUDIES14.1 Tadalafil for Use as Needed for ED - The efficacy and safety of tadalafil in the treatment of erectile dysfunction has been evaluated in 22 clinical trials of up to 24-weeks duration ...
14.1 Tadalafil for Use as Needed for ED
The efficacy and safety of tadalafil in the treatment of erectile dysfunction has been evaluated in 22 clinical trials of up to 24-weeks duration, involving over 4,000 patients. Tadalafil, when taken as needed up to once per day, was shown to be effective in improving erectile function in men with erectile dysfunction (ED).
Tadalafil was studied in the general ED population in 7 randomized, multicenter, double-blinded, placebo-controlled, parallel-arm design, primary efficacy and safety studies of 12-weeks duration. Two of these studies were conducted in the United States and 5 were conducted in centers outside the U.S. Additional efficacy and safety studies were performed in ED patients with diabetes mellitus and in patients who developed ED status post bilateral nerve-sparing radical prostatectomy.
In these 7 trials, tadalafil was taken as needed, at doses ranging from 2.5 to 20 mg, up to once per day. Patients were free to choose the time interval between dose administration and the time of sexual attempts. Food and alcohol intake were not restricted.
Several assessment tools were used to evaluate the effect of tadalafil on erectile function. The 3 primary outcome measures were the Erectile Function (EF) domain of the International Index of Erectile Function (IIEF) and Questions 2 and 3 from Sexual Encounter Profile (SEP). The IIEF is a 4-week recall questionnaire that was administered at the end of a treatment-free baseline period and subsequently at follow-up visits after randomization. The IIEF EF domain has a 30-point total score, where higher scores reflect better erectile function. SEP is a diary in which patients recorded each sexual attempt made throughout the study. SEP Question 2 asks, "Were you able to insert your penis into the partner's vagina?" SEP Question 3 asks, "Did your erection last long enough for you to have successful intercourse?" The overall percentage of successful attempts to insert the penis into the vagina (SEP2) and to maintain the erection for successful intercourse (SEP3) is derived for each patient.
Results in ED Population in U.S. Trials— The 2 primary U.S. efficacy and safety trials included a total of 402 men with erectile dysfunction, with a mean age of 59 years (range 27 to 87 years). The population was 78% White, 14% Black, 7% Hispanic, and 1% of other ethnicities, and included patients with ED of various severities, etiologies (organic, psychogenic, mixed), and with multiple co-morbid conditions, including diabetes mellitus, hypertension, and other cardiovascular disease. Most (>90%) patients reported ED of at least 1-year duration. Study A was conducted primarily in academic centers. Study B was conducted primarily in community-based urology practices. In each of these 2 trials, tadalafil 20 mg showed clinically meaningful and statistically significant improvements in all 3 primary efficacy variables ( seeTable 11). The treatment effect of tadalafil did not diminish over time.
Table 11: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in the Two Primary US Trials
Study A
Study B
Placebo
Tadalafil
20 mg
Placebo
Tadalafil20 mg
(N=49)
(N=146)
p-value
(N=48)
(N=159)
p-value
EF Domain Score
Endpoint
13.5
19.5
13.6
22.5
Change from baseline
-0.2
6.9
<.001
0.3
9.3
<.001
Insertion of Penis (SEP2)
Endpoint
39%
62%
43%
77%
Change from baseline
2%
26%
<.001
2%
32%
<.001
Maintenance of Erection (SEP3)
Endpoint
25%
50%
23%
64%
Change from baseline
5%
34%
<.001
4%
44%
<.001
Results in General ED Population in Trials Outside the U.S.— The 5 primary efficacy and safety studies conducted in the general ED population outside the U.S. included 1,112 patients, with a mean age of 59 years (range 21 to 82 years). The population was 76% White, 1% Black, 3% Hispanic, and 20% of other ethnicities, and included patients with ED of various severities, etiologies (organic, psychogenic, mixed), and with multiple co-morbid conditions, including diabetes mellitus, hypertension, and other cardiovascular disease. Most (90%) patients reported ED of at least 1-year duration. In these 5 trials, tadalafil 5, 10, and 20 mg showed clinically meaningful and statistically significant improvements in all 3 primary efficacy variables ( seeTables 12, 13 and 14). The treatment effect of tadalafil did not diminish over time.
Table 12: Mean Endpoint and Change from Baseline for the EF Domain of the IIEF in the General ED Population in Five Primary Trials Outside the U.S.
Placebo
Tadalafil
5 mg
Tadalafil
10 mg
Tadalafil
20 mg
Study C
Endpoint [Change from baseline]
15 [0.7]
17.9 [4]
20 [5.6]
p=.006
p<.001
Study D
Endpoint [Change from baseline]
14.4 [1.1]
17.5 [5.1]
20.6 [6]
p=.002
p<.001
Study E
Endpoint [Change from baseline]
18.1 [2.6]
22.6 [8.1]
25 [8]
p<.001
p<.001
Study F a
Endpoint [Change from baseline]
12.7 [-1.6]
22.8 [6.8]
p<.001
Study G
Endpoint [Change from baseline]
14.5 [-0.9]
21.2 [6.6]
23.3 [8]
p<.001
p<.001
Table 13: Mean Post-Baseline Success Rate and Change from Baseline for SEP Question 2 ("Were you able to insert your penis into the partner's vagina?") in the General ED Population in Five Pivotal Trials Outside the U.S.
Placebo
Tadalafil
5 mg
Tadalafil
10 mg
Tadalafil
20 mg
Study C
Endpoint [Change from baseline]
49% [6%]
57% [15%]
73% [29%]
p=.063
p<.001
Study D
Endpoint [Change from baseline]
46% [2%]
56% [18%]
68% [15%]
p=.008
p<.001
Study E
Endpoint [Change from baseline]
55% [10%]
77% [35%]
85% [35%]
p<.001
p<.001
Study F a
Endpoint [Change from baseline]
42% [-8%]
81% [27%]
p<.001
Study G
Endpoint [Change from baseline]
45% [-6%]
73% [21%]
76% [21%]
p<.001
p<.001
Table 14: Mean Post-Baseline Success Rate and Change from Baseline for SEP Question 3 ("Did your erection last long enough for you to have successful intercourse?") in the General ED Population in Five Pivotal Trials Outside the U.S.
aTreatment duration in Study F was 6 months
Placebo
Tadalafil
5 mg
Tadalafil
10 mg
Tadalafil
20 mg
Study C
Endpoint [Change from baseline]
26% [4%]
38% [19%]
58% [32%]
p=.040
p<.001
Study D
Endpoint [Change from baseline]
28% [4%]
42% [24%]
51% [26%]
p<.001
p<.001
Study E
Endpoint [Change from baseline]
43% [15%]
70% [48%]
78% [50%]
p<.001
p<.001
Study F a
Endpoint [Change from baseline]
27% [1%]
74% [40%]
p<.001
Study G
Endpoint [Change from baseline]
32% [5%]
57% [33%]
62% [29%]
p<.001
p<.001
In addition, there were improvements in EF domain scores, success rates based upon SEP Questions 2 and 3, and patient-reported improvement in erections across patients with ED of all degrees of disease severity while taking tadalafil, compared to patients on placebo.
Therefore, in all 7 primary efficacy and safety studies, tadalafil showed statistically significant improvement in patients' ability to achieve an erection sufficient for vaginal penetration and to maintain the erection long enough for successful intercourse, as measured by the IIEF questionnaire and by SEP diaries.
Efficacy Results in ED Patients with Diabetes Mellitus— Tadalafil was shown to be effective in treating ED in patients with diabetes mellitus. Patients with diabetes were included in all 7 primary efficacy studies in the general ED population (N=235) and in one study that specifically assessed tadalafil in ED patients with type 1 or type 2 diabetes (N=216). In this randomized, placebo-controlled, double-blinded, parallel-arm design prospective trial, tadalafil demonstrated clinically meaningful and statistically significant improvement in erectile function, as measured by the EF domain of the IIEF questionnaire and Questions 2 and 3 of the SEP diary ( seeTable 15).
Table 15: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in a Study in ED Patients with Diabetes
Placebo
Tadalafil
10 mg
Tadalafil
20 mg
(N=71)
(N=73)
(N=72)
p-value
EF Domain Score
Endpoint [Change from baseline]
12.2 [0.1]
19.3 [6.4]
18.7 [7.3]
<.001
Insertion of Penis (SEP2)
Endpoint [Change from baseline]
30% [-4%]
57% [22%]
54% [23%]
<.001
Maintenance of Erection (SEP3)
Endpoint [Change from baseline]
20% [2%]
48% [28%]
42% [29%]
<.001
Efficacy Results in ED Patients following Radical Prostatectomy— Tadalafil was shown to be effective in treating patients who developed ED following bilateral nerve-sparing radical prostatectomy. In 1 randomized, placebo-controlled, double-blinded, parallel-arm design prospective trial in this population (N=303), tadalafil demonstrated clinically meaningful and statistically significant improvement in erectile function, as measured by the EF domain of the IIEF questionnaire and Questions 2 and 3 of the SEP diary ( seeTable 16).
Table 16: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in a Study in Patients who Developed ED Following Bilateral Nerve-Sparing Radical Prostatectomy
Placebo
Tadalafil
20 mg
(N=102)
(N=201)
p-value
EF Domain Score
Endpoint [Change from baseline]
13.3 [1.1]
17.7 [5.3]
<.001
Insertion of Penis (SEP2)
Endpoint [Change from baseline]
32% [2%]
54% [22%]
<.001
Maintenance of Erection (SEP3)
Endpoint [Change from baseline]
19% [4%]
41% [23%]
<.001
Results in Studies to Determine the Optimal Use of Tadalafil— Several studies were conducted with the objective of determining the optimal use of tadalafil in the treatment of ED. In one of these studies, the percentage of patients reporting successful erections within 30 minutes of dosing was determined. In this randomized, placebo-controlled, double-blinded trial, 223 patients were randomized to placebo, tadalafil 10, or 20 mg. Using a stopwatch, patients recorded the time following dosing at which a successful erection was obtained. A successful erection was defined as at least 1 erection in 4 attempts that led to successful intercourse. At or prior to 30 minutes, 35% (26/74), 38% (28/74), and 52% (39/75) of patients in the placebo, 10, and 20 mg groups, respectively, reported successful erections as defined above.
Two studies were conducted to assess the efficacy of tadalafil at a given timepoint after dosing, specifically at 24 hours and at 36 hours after dosing.
In the first of these studies, 348 patients with ED were randomized to placebo or tadalafil 20 mg. Patients were encouraged to make 4 total attempts at intercourse; 2 attempts were to occur at 24 hours after dosing and 2 completely separate attempts were to occur at 36 hours after dosing. The results demonstrated a difference between the placebo group and the tadalafil group at each of the pre-specified timepoints. At the 24-hour timepoint, (more specifically, 22 to 26 hours), 53/144 (37%) patients reported at least 1 successful intercourse in the placebo group versus 84/138 (61%) in the tadalafil 20 mg group. At the 36-hour time point (more specifically, 33 to 39 hours), 49/133 (37%) of patients reported at least 1 successful intercourse in the placebo group versus 88/137 (64%) in the tadalafil 20 mg group.
In the second of these studies, a total of 483 patients were evenly randomized to 1 of 6 groups: 3 different dosing groups (placebo, tadalafil 10, or 20 mg) that were instructed to attempt intercourse at 2 different times (24 and 36 hours post-dosing). Patients were encouraged to make 4 separate attempts at their assigned dose and assigned timepoint. In this study, the results demonstrated a statistically significant difference between the placebo group and the tadalafil groups at each of the pre-specified time points. At the 24-hour time point, the mean, per patient percentage of attempts resulting in successful intercourse were 42, 56, and 67% for the placebo, tadalafil 10, and 20 mg groups, respectively. At the 36-hour timepoint, the mean, per-patient percentage of attempts resulting in successful intercourse were 33, 56, and 62% for placebo, tadalafil 10, and 20 mg groups, respectively.
14.2 Tadalafil for Once Daily Use for ED
The efficacy and safety of tadalafil for once daily use in the treatment of erectile dysfunction has been evaluated in 2 clinical trials of 12-weeks duration and 1 clinical trial of 24-weeks duration, involving a total of 853 patients. Tadalafil, when taken once daily, was shown to be effective in improving erectile function in men with erectile dysfunction (ED).
Tadalafil was studied in the general ED population in 2 randomized, multicenter, double-blinded, placebo-controlled, parallel-arm design, primary efficacy and safety studies of 12- and 24-weeks duration, respectively. One of these studies was conducted in the United States and one was conducted in centers outside the U.S. An additional efficacy and safety study was performed in ED patients with diabetes mellitus. Tadalafil was taken once daily at doses ranging from 2.5 to 10 mg. Food and alcohol intake were not restricted. Timing of sexual activity was not restricted relative to when patients took tadalafil.
Results in General ED Population— The primary U.S. efficacy and safety trial included a total of 287 patients, with a mean age of 59 years (range 25 to 82 years). The population was 86% White, 6% Black, 6% Hispanic, and 2% of other ethnicities, and included patients with ED of various severities, etiologies (organic, psychogenic, mixed), and with multiple co-morbid conditions, including diabetes mellitus, hypertension, and other cardiovascular disease. Most (>96%) patients reported ED of at least 1-year duration.
The primary efficacy and safety study conducted outside the U.S. included 268 patients, with a mean age of 56 years (range 21 to 78 years). The population was 86% White, 3% Black, 0.4% Hispanic, and 10% of other ethnicities, and included patients with ED of various severities, etiologies (organic, psychogenic, mixed), and with multiple co-morbid conditions, including diabetes mellitus, hypertension, and other cardiovascular disease. Ninety-three percent of patients reported ED of at least 1-year duration.
In each of these trials, conducted without regard to the timing of dose and sexual intercourse, tadalafil demonstrated clinically meaningful and statistically significant improvement in erectile function, as measured by the EF domain of the IIEF questionnaire and Questions 2 and 3 of the SEP diary ( seeTable 17). When taken as directed, tadalafil was effective at improving erectile function.
In the 6 month double-blind study, the treatment effect of tadalafil did not diminish over time.
Table 17: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in the Two Tadalafil for Once Daily Use Studies
aTwenty-four-week study conducted in the U.S.
bTwelve-week study conducted outside the U.S.
cStatistically significantly different from placebo.
Study H a
Study I b
Placebo
Tadalafil
2.5 mg
Tadalafil
5 mg
Placebo
Tadalafil
5 mg
(N=94)
(N=96)
(N=97)
p-value
(N=54)
(N=109)
p-value
EF Domain Score
Endpoint
14.6
19.1
20.8
15
22.8
Change from baseline
1.2
6.1 c
7 c
<.001
0.9
9.7 c
<.001
Insertion of Penis (SEP2)
Endpoint
51%
65%
71%
52%
79%
Change from baseline
5%
24% c
26% c
<.001
11%
37% c
<.001
Maintenance of Erection (SEP3)
Endpoint
31%
50%
57%
37%
67%
Change from baseline
10%
31% c
35% c
<.001
13%
46% c
<.001
Efficacy Results in ED Patients with Diabetes Mellitus— Tadalafil for once daily use was shown to be effective in treating ED in patients with diabetes mellitus. Patients with diabetes were included in both studies in the general ED population (N=79). A third randomized, multicenter, double-blinded, placebo-controlled, parallel-arm design trial included only ED patients with type 1 or type 2 diabetes (N=298). In this third trial, tadalafil demonstrated clinically meaningful and statistically significant improvement in erectile function, as measured by the EF domain of the IIEF questionnaire and Questions 2 and 3 of the SEP diary ( seeTable 18).
Table 18: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in a Tadalafil Tablets for Once Daily Use Study in ED Patients with Diabetes
aStatistically significantly different from placebo.
Placebo
Tadalafil
2.5 mg
Tadalafil
5 mg
(N=100)
(N=100)
(N=98)
p-value
EF Domain Score
Endpoint
14.7
18.3
17.2
Change from baseline
1.3
4.8 a
4.5 a
<.001
Insertion of Penis (SEP2)
Endpoint
43%
62%
61%
Change from baseline
5%
21% a
29% a
<.001
Maintenance of Erection (SEP3)
Endpoint
28%
46%
41%
Change from baseline
8%
26% a
25% a
<.001
14.3 Tadalafil 5 mg for Once Daily Use for Benign Prostatic Hyperplasia (BPH)
The efficacy and safety of tadalafil for once daily use for the treatment of the signs and symptoms of BPH was evaluated in 3 randomized, multinational, double-blinded, placebo-controlled, parallel-design, efficacy and safety studies of 12 weeks duration. Two of these studies were in men with BPH and one study was specific to men with both ED and BPH [see Clinical Studies ( 14.4)]. The first study (Study J) randomized 1,058 patients to receive either tadalafil 2.5 mg, 5 mg, 10 mg or 20 mg for once daily use or placebo. The second study (Study K) randomized 325 patients to receive either tadalafil 5 mg for once daily use or placebo. The full study population was 87% White, 2% Black, 11% other races; 15% was of Hispanic ethnicity. Patients with multiple co-morbid conditions such as diabetes mellitus, hypertension, and other cardiovascular disease were included.
The primary efficacy endpoint in the two studies that evaluated the effect of tadalafil for the signs and symptoms of BPH was the International Prostate Symptom Score (IPSS), a four week recall questionnaire that was administered at the beginning and end of a placebo run-in period and subsequently at follow-up visits after randomization. The IPSS assesses the severity of irritative (frequency, urgency, nocturia) and obstructive symptoms (incomplete emptying, stopping and starting, weak stream, and pushing or straining), with scores ranging from 0 to 35; higher numeric scores representing greater severity. Maximum urinary flow rate (Q max), an objective measure of urine flow, was assessed as a secondary efficacy endpoint in Study J and as a safety endpoint in Study K.
The results for BPH patients with moderate to severe symptoms and a mean age of 63.2 years (range 44 to 87) who received either tadalafil 5 mg for once daily use or placebo (N=748) in Studies J and K are shown in Table 19 and Figures 5 and 6, respectively.
In each of these 2 trials, tadalafil 5 mg for once daily use resulted in statistically significant improvement in the total IPSS compared to placebo. Mean total IPSS showed a decrease starting at the first scheduled observation (4 weeks) in Study K and remained decreased through 12 weeks.
Table 19: Mean IPSS Changes in BPH Patients in Two Tadalafil for Once Daily Use Studies
Study J
Study K
Placebo
Tadalafil
5 mg
Placebo
Tadalafil
5 mg
(N=205)
(N=205)
p-value
(N=164)
(N=160)
p-value
Total Symptom Score (IPSS)
Baseline
17.1
17.3
16.6
17.1
Change from Baseline to Week 12
-2.2
-4.8
<.001
-3.6
-5.6
.004

In Study J, the effect of tadalafil 5 mg once daily on maximum urinary flow rate (Q max) was evaluated as a secondary efficacy endpoint. Mean Q maxincreased from baseline in both the treatment and placebo groups (tadalafil 5 mg: 1.6 mL/sec, placebo: 1.2 mL/sec); however, these changes were not significantly different between groups.
In Study K, the effect of tadalafil 5 mg once daily on Q maxwas evaluated as a safety endpoint. Mean Q maxincreased from baseline in both the treatment and placebo groups (tadalafil 5 mg: 1.6 mL/sec, placebo: 1.1 mL/sec); however, these changes were not significantly different between groups.
Efficacy Results in Patients with BPH initiating Tadalafil and Finasteride– Tadalafil for once daily use initiated together with finasteride was shown to be effective in treating the signs and symptoms of BPH in men with an enlarged prostate (>30 cc) for up to 26 weeks. This additional double-blinded, parallel-design study of 26 weeks duration randomized 696 men to initiate either tadalafil 5 mg with finasteride 5 mg or placebo with finasteride 5 mg. The study population had a mean age of 64 years (range 46 to 86). Patients with multiple co-morbid conditions such as erectile dysfunction, diabetes mellitus, hypertension, and other cardiovascular disease were included.
Tadalafil with finasteride demonstrated statistically significant improvement in the signs and symptoms of BPH compared to placebo with finasteride, as measured by the total IPSS at 12 weeks, the primary study endpoint ( seeTable 20). Key secondary endpoints demonstrated improvement in total IPSS starting at the first scheduled observation at week 4 (tadalafil -4, placebo -2.3: p<.001) and the score remained decreased through 26 weeks (tadalafil -5.5, placebo -4.5; p=.022). However, the magnitude of the treatment difference between placebo/finasteride and tadalafil/finasteride decreased from 1.7 points at Week 4 to 1 point at Week 26, as shown in Table 20 and in Figure 7. The incremental benefit of tadalafil beyond 26 weeks is unknown.
Table 20: Mean Total IPSS Changes in BPH Patients in a Tadalafil for Once Daily Use Study Together with Finasteride
Placebo and finasteride
5 mg
Tadalafil 5mg and finasteride
5 mg
Treatment difference
n
(N=350) a
n
(N=345) a
p-value b
Total Symptom Score (IPSS)
Baseline c
349
17.4
344
17.1
Change from Baseline to Week 4 b
340
-2.3
330
-4
-1.7
<.001
Change from Baseline to Week 12 b
318
-3.8
317
-5.2
-1.4
.001
Change from Baseline to Week 26 b
295
-4.5
308
-5.5
-1
.022
aOverall ITT population.
bMixed model for repeated measurements.
cUnadjusted mean.
Figure 7: Mean Total IPSS Changes By Visit in BPH Patients Taking Tadalafil for Once Daily Use Together With Finasteride
In the 404 patients who had both ED and BPH at baseline, changes in erectile function were assessed as key secondary endpoints using the EF domain of the IIEF questionnaire. Tadalafil with finasteride (N=203) was compared to placebo with finasteride (N=201). A statistically significant improvement from baseline (tadalafil/finasteride 13.7, placebo/finasteride 15.1) was observed at week 4 (tadalafil/finasteride 3.7, placebo/finasteride -1.1; p<.001), week 12 (tadalafil/finasteride 4.7, placebo/finasteride 0.6; p<.001), and week 26 (tadalafil/finasteride 4.7, placebo/finasteride 0; p<.001).
Close14.4 Tadalafil 5 mg for Once Daily Use for ED and BPH
The efficacy and safety of tadalafil for once daily use for the treatment of ED, and the signs and symptoms of BPH, in patients with both conditions was evaluated in one placebo-controlled, multinational, double-blind, parallel-arm study which randomized 606 patients to receive either tadalafil 2.5 mg, 5 mg, for once daily use or placebo. ED severity ranged from mild to severe and BPH severity ranged from moderate to severe. The full study population had a mean age of 63 years (range 45 to 83) and was 93% White, 4% Black, 3% other races; 16% were of Hispanic ethnicity. Patients with multiple co-morbid conditions such as diabetes mellitus, hypertension, and other cardiovascular disease were included.
In this study, the co-primary endpoints were total IPSS and the Erectile Function (EF) domain score of the International Index of Erectile Function (IIEF). One of the key secondary endpoints in this study was Question 3 of the Sexual Encounter Profile diary (SEP3). Timing of sexual activity was not restricted relative to when patients took tadalafil.
The efficacy results for patients with both ED and BPH, who received either tadalafil 5 mg for once daily use or placebo (N=408) are shown in Tables 21 and 22 and Figure 8.
Tadalafil 5 mg for once daily use resulted in statistically significant improvements in the total IPSS and in the EF domain of the IIEF questionnaire. Tadalafil 5 mg for once daily use also resulted in statistically significant improvement in SEP3. Tadalafil 2.5 mg did not result in statistically significant improvement in the total IPSS.
Table 21: Mean IPSS and IIEF EF Domain Changes in the Tadalafil 5 mg for Once Daily Use Study in Patients with ED and BPH
Placebo
Tadalafil 5 mg
p-value
Total Symptom Score (IPSS)
(N=193)
(N=206)
Baseline
18.2
18.5
Change from Baseline to Week 12
-3.8
-6.1
<.001
EF Domain Score (IIEF EF)
(N=188)
(N=202)
Baseline
15.6
16.5
Endpoint
17.6
22.9
Change from Baseline to Week 12
1.9
6.5
<.001
Table 22: Mean SEP Question 3 Changes in the Tadalafil 5 mg for Once Daily Use Study in Patients with ED and BPH
Placebo
Tadalafil5 mg
(N=187)
(N=199)
p-value
Maintenance of Erection (SEP3)
Baseline
36%
43%
Endpoint
48%
72%
Change from Baseline to Week 12
12%
32%
<.001
Tadalafil for once daily use resulted in improvement in the IPSS total score at the first scheduled observation (week 2) and throughout the 12 weeks of treatment ( seeFigure 8).

Figure 8: Mean IPSS Changes in ED/BPH Patients by Visit in Study L
In this study, the effect of tadalafil 5 mg once daily on Q maxwas evaluated as a safety endpoint. Mean Q maxincreased from baseline in both the treatment and placebo groups (tadalafil tablets 5 mg: 1.6 mL/sec, placebo: 1.2 mL/sec); however, these changes were not significantly different between groups. -
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Tadalafil tablets USP, are supplied as follows: Tadalafil tablets USP, 2.5 mg are blue color, round shaped, biconvex, film-coated tablets debossed with '122' on one side ...
16.1 How Supplied
Tadalafil tablets USP, are supplied as follows:
Tadalafil tablets USP, 2.5 mg are blue color, round shaped, biconvex, film-coated tablets debossed with '122' on one side and 'E' on the other side.
Bottles of 30 tablets NDC 72865-104-30
Tadalafil tablets USP, 5 mg are white color, round shaped, biconvex, film-coated tablets debossed with '123' on one side and 'E' on the other side.
Bottles of 30 tablets NDC 72865-106-30
Tadalafil tablets USP, 10 mg are white color, capsule shaped, biconvex, film-coated tablets debossed with '124' on one side and 'E' on the other side.
Bottles of 30 tablets NDC 72865-107-30
Tadalafil tablets USP, 20 mg are white color, capsule shaped, biconvex, film-coated tablets debossed with '125' on one side and 'E' on the other side.
Bottles of 30 tablets NDC 72865-108-30
Close16.2 Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight container.
Keep out of reach of children. -
17 PATIENT COUNSELING INFORMATION17.1 Nitrates - "See FDA-approved patient labeling (Patient Information)" Physicians should discuss with patients the contraindication of tadalafil tablets with regular and/or ...
17.1 Nitrates
"See FDA-approved patient labeling (Patient Information)"
Physicians should discuss with patients the contraindication of tadalafil tablets with regular and/or intermittent use of organic nitrates. Patients should be counseled that concomitant use of tadalafil tablets with nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke.
Physicians should discuss with patients the appropriate action in the event that they experience anginal chest pain requiring nitroglycerin following intake of tadalafil tablets. In such a patient, who has taken tadalafil tablets, where nitrate administration is deemed medically necessary for a life-threatening situation, at least 48 hours should have elapsed after the last dose of tadalafil tablets before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring. Therefore, patients who experience anginal chest pain after taking tadalafil tablets should seek immediate medical attention [see Contraindications ( 4.1) and Warnings and Precautions ( 5.1)].
17.2 Guanylate Cyclase (GC) Stimulators
Physicians should discuss with patients the contraindication of tadalafil tablets with any use of a GC stimulator, such as riociguat, for pulmonary arterial hypertension. Patients should be counseled that the concomitant use of tadalafil tablets with GC stimulators may cause blood pressure to drop to an unsafe level.
17.3 Cardiovascular Considerations
Physicians should consider the potential cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Physicians should advise patients who experience symptoms upon initiation of sexual activity to refrain from further sexual activity and seek immediate medical attention [see Warnings and Precautions ( 5.1)].
17.4 Concomitant Use with Drugs Which Lower Blood Pressure
Physicians should discuss with patients the potential for tadalafil tablets to augment the blood-pressure-lowering effect of alpha-blockers, and antihypertensive medications [see Warnings and Precautions ( 5.6), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.2)].
17.5 Potential for Drug Interactions When Taking Tadalafil Tablets for Once Daily Use
Physicians should discuss with patients the clinical implications of continuous exposure to tadalafil when prescribing tadalafil tablets for once daily use, especially the potential for interactions with medications (e.g., nitrates, alpha-blockers, antihypertensives and potent inhibitors of cytochrome P450 3A4) and with substantial consumption of alcohol. [see Dosage and Administration ( 2.7), Warnings and Precautions ( 5.6), Drug Interactions ( 7.1, 7.2), Clinical Pharmacology ( 12.2), and Clinical Studies ( 14.2)].
17.6 Priapism
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Physicians should advise patients who have an erection lasting greater than 4 hours, whether painful or not, to seek emergency medical attention.
17.7 Sudden Loss of Vision
Physicians should advise patients to stop use of all PDE5 inhibitors, including tadalafil tablets, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision, including possible permanent loss of vision, that has been reported rarely postmarketing in temporal association with the use of all PDE5 inhibitors. Physicians should discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye. Physicians should also discuss with patients the increased risk of NAION among the general population in patients with a "crowded" optic disc, although evidence is insufficient to support screening of prospective users of PDE5 inhibitors, including tadalafil tablets, for this uncommon condition [see Warnings and Precautions ( 5.4) and Adverse Reactions ( 6.2)].
17.8 Sudden Hearing Loss
Physicians should advise patients to stop taking PDE5 inhibitors, including tadalafil tablets, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including tadalafil tablets. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions ( 6.1, 6.2)].
17.9 Alcohol
Patients should be made aware that both alcohol and tadalafil tablets, a PDE5 inhibitor, act as mild vasodilators. When mild vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., 5 units or greater) in combination with tadalafil tablets can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Warnings and Precautions ( 5.9), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.2)].
17.10 Sexually Transmitted Disease
The use of tadalafil tablets offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV) should be considered.
Close17.11 Recommended Administration
Physicians should instruct patients on the appropriate administration of tadalafil tablets to allow optimal use.
For tadalafil tablets for use as needed in men with ED, patients should be instructed to take one tablet at least 30 minutes before anticipated sexual activity. In most patients, the ability to have sexual intercourse is improved for up to 36 hours.
For tadalafil tablets for once daily use in men with ED or ED/BPH, patients should be instructed to take one tablet at approximately the same time every day without regard for the timing of sexual activity. Tadalafil tablet is effective at improving erectile function over the course of therapy.
For tadalafil tablets for once daily use in men with BPH, patients should be instructed to take one tablet at approximately the same time every day.
The brands listed are trademarks of their respective owners and are not trademarks of Evaric Pharmaceuticals Inc.
Revised Date:11/24
Manufactured for:
XLCare Pharmaceuticals, Inc., 242 South Culver Street,
Suite 202 Lawrenceville, GA 30046.
Manufactured by:
Evaric Pharmaceuticals Inc.
155 Commerce Drive,
Hauppauge, New York 11788,
United States (USA). -
Patient InformationTadalafil (ta DAL a fil) tablets, USP - Read this important information before you start taking tadalafil tablets and each time you get a refill. There may be new information. You ...
Tadalafil (ta DAL a fil)
tablets, USP
Read this important information before you start taking tadalafil tablets and each time you get a refill. There may be new information. You may also find it helpful to share this information with your partner. This information does not take the place of talking with your healthcare provider. You and your healthcare provider should talk about tadalafil tablets when you start taking it and at regular checkups. If you do not understand the information, or have questions, talk with your healthcare provider or pharmacist.
Close
What Is The Most Important Information I Should Know About Tadalafil Tablets?
Tadalafil tablets can cause your blood pressure to drop suddenly to an unsafe level if it is taken with certain other medicines. You could get dizzy, faint, or have a heart attack or stroke. Never take tadalafil tablets with any nitrate or guanylate cyclase stimulator medicines.
Do not take tadalafil tablets if you take any medicines called "nitrates".Nitrates are commonly used to treat angina. Angina is a symptom of heart disease and can cause pain in your chest, jaw, or down your arm.
• Medicines called nitrates include nitroglycerin that is found in tablets, sprays, ointments, pastes, or patches. Nitrates can also be found in other medicines such as isosorbide dinitrate or isosorbide mononitrate. Some recreational drugs called "poppers" also contain nitrates, such as amyl nitrite and butyl nitrite.
Do not take tadalafil tablets if you take medicines called guanylate cyclase stimulators which include:
• Riociguat (Adempas ®) a medicine that treats pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension.
Ask your healthcare provider or pharmacist if you are not sure if any of your medicines are nitrates or guanylate cyclase stimulators, such as riociguat.
(See "Who Should Not Take Tadalafil Tablets?")
Tell all of your healthcare providers that you take tadalafil tablets. If you need emergency medical care for a heart problem, it will be important for your healthcare provider to know when you last took tadalafil tablets.
After taking a single tablet, some of the active ingredient of tadalafil tablets remains in your body for more than 2 days.The active ingredient can remain longer if you have problems with your kidneys or liver, or you are taking certain other medications (see "Can Other Medicines Affect Tadalafil Tablets?').
Stop sexual activity and get medical help right away if you get symptoms such as chest pain, dizziness, or nausea during sex. Sexual activity can put an extra strain on your heart, especially if your heart is already weak from a heart attack or heart disease.
See also "What Are The Possible Side Effects Of Tadalafil Tablets?"
What Are Tadalafil Tablets?
Tadalafil tablet is a prescription medicine taken by mouth for the treatment of:
• men with erectile dysfunction (ED)
• men with symptoms of benign prostatic hyperplasia (BPH)
• men with both ED and BPH
Tadalafil tablets for the Treatment of ED
ED is a condition where the penis does not fill with enough blood to harden and expand when a man is sexually excited, or when he cannot keep an erection. A man who has trouble getting or keeping an erection should see his healthcare provider for help if the condition bothers him. Tadalafil tablets helps increase blood flow to the penis and may help men with ED get and keep an erection satisfactory for sexual activity. Once a man has completed sexual activity, blood flow to his penis decreases, and his erection goes away.
Some form of sexual stimulation is needed for an erection to happen with tadalafil tablets.
Tadalafil tablets does not:
• cure ED
• increase a man’s sexual desire
• protect a man or his partner from sexually transmitted diseases, including HIV. Speak to your healthcare provider about ways to guard against sexually transmitted diseases.
• serve as a male form of birth control
Tadalafil tablets is only for men over the age of 18, including men with diabetes or who have undergone prostatectomy.
Tadalafil Tablets for the Treatment of Symptoms of BPH
BPH is a condition that happens in men, where the prostate gland enlarges which can cause urinary symptoms.
Tadalafil Tablets for the Treatment of ED and Symptoms of BPH
ED and symptoms of BPH may happen in the same person and at the same time. Men who have both ED and symptoms of BPH may take tadalafil tablets for the treatment of both conditions.
Tadalafil tablets are not for women or children.
Tadalafil tablets must be used only under a healthcare provider's care.
Who Should Not Take Tadalafil Tablets?
Do not take tadalafil tablets if you:
• take any medicines called "nitrates".
• use recreational drugs called “poppers” like amyl nitrite and butyl nitrite. (See "What Is The Most Important Information I Should Know About Tadalafil Tablets?")
• take any medicines called guanylate cyclase stimulators, such as riociguat.
• are allergic to tadalafil tablets or ADCIRCA ®, or any of its ingredients. See the end of this leaflet for a complete list of ingredients in tadalafil tablets. Symptoms of an allergic reaction may include:
• rash
• hives
• swelling of the lips, tongue, or throat
• difficulty breathing or swallowing
Call your healthcare provider or get help right away if you have any of the symptoms of an allergic reaction listed above.
What Should I Tell My Healthcare Provider Before Taking Tadalafil Tablets?
Tadalafil tablets are not right for everyone. Only your healthcare provider and you can decide if tadalafil tablet is right for you.Before taking tadalafil tablets, tell your healthcare provider about all your medical problems, including if you:
• have heart problemssuch as angina, heart failure, irregular heartbeats, or have had a heart attack. Ask your healthcare provider if it is safe for you to have sexual activity. You should not take tadalafil tablets if your healthcare provider has told you not to have sexual activity because of your health problems.
• have pulmonary hypertension
• have low blood pressure orhave high blood pressure that is not controlled
• have had a stroke
• have liver problems
• have kidney problems or require dialysis
• have retinitis pigmentosa,a rare genetic (runs in families) eye disease
• have ever had severe vision loss, including a condition called NAION
• have stomach ulcers
• have a bleeding problem
• have a deformed penis shapeor Peyronie's disease
• have had an erection that lasted more than 4 hours
• have blood cell problemssuch as sickle cell anemia, multiple myeloma, or leukemia
Can Other Medicines Affect Tadalafil Tablets?
Tell your healthcare provider about all the medicines you take including prescription and non-prescription medicines, vitamins, and herbal supplements. Tadalafil tablets and other medicines may affect each other. Always check with your healthcare provider before starting or stopping any medicines. Especially tell your healthcare provider if you take any of the following*:
• medicines called nitrates (see "What Is The Most Important Information I Should Know About Tadalafil Tablets?")
• medicines called guanylate cyclase stimulators, such as riociguat (Adempas ®), used to treat pulmonary hypertension
• medicines called alpha blockers. These include Hytrin ®(terazosin HCl), Flomax ®(tamsulosin HCl), Cardura ®(doxazosin mesylate), Minipress ®(prazosin HCl), Uroxatral ®(alfuzosin HCl), Jalyn ®(dutasteride and tamsulosin HCl) or Rapaflo ®(silodosin). Alpha-blockers are sometimes prescribed for prostate problems or high blood pressure. If tadalafil tablets is taken with certain alpha blockers, your blood pressure could suddenly drop. You could get dizzy or faint.
• other medicines to treat high blood pressure (hypertension)
• medicines called HIV protease inhibitors, such as ritonavir (Norvir ®, Kaletra ®)
• some types of oral antifungals such as ketoconazole (Nizoral ®), itraconazole (Sporanox ®)
• some types of antibiotics such as clarithromycin (Biaxin ®), telithromycin (Ketek ®), erythromycin (several brand names exist. Please consult your healthcare provider to determine if you are taking this medicine).
• other medicines or treatments for ED.
• Tadalafil tablets is also marketed as ADCIRCA for the treatment of pulmonary arterial hypertension. Do not take both tadalafil tablets and ADCIRCA. Do not take sildenafil citrate (Revatio ®) with tadalafil tablets.
How Should I Take Tadalafil Tablets?
• Take tadalafil tablets exactly as your healthcare provider prescribes it. Your healthcare provider will prescribe the dose that is right for you.
• Some men can only take a low dose of tadalafil tablets or may have to take it less often, because of medical conditions or medicines they take.
• Do not change your dose or the way you take tadalafil tablets without talking to your healthcare provider. Your healthcare provider may lower or raise your dose, depending on how your body reacts to tadalafil tablets and your health condition.
• Tadalafil tablets may be taken with or without meals.
• If you take too much tadalafil tablets, call your healthcare provider or emergency room right away.
How Should I Take Tadalafil Tablets for Symptoms of BPH?
For symptoms of BPH, tadalafil tablet is taken once daily.
• Do not take tadalafil tablets more than one time each day.
• Take one tadalafil tablet every day at about the same time of day.
• If you miss a dose, you may take it when you remember but do not take more than one dose per day.
How Should I Take Tadalafil Tablets for ED?
For ED, there are two ways to take tadalafil tablets - either for use as needed OR for use once daily.
Tadalafil tablets for use as needed:
• Do not take tadalafil tablets more than one time each day.
• Take one tadalafil tablet before you expect to have sexual activity. You may be able to have sexual activity at 30 minutes after taking tadalafil tablets and up to 36 hours after taking it. You and your healthcare provider should consider this in deciding when you should take tadalafil tablets before sexual activity. Some form of sexual stimulation is needed for an erection to happen with tadalafil tablets.
• Your healthcare provider may change your dose of tadalafil tablets depending on how you respond to the medicine, and on your health condition.
OR
Tadalafil tablets for once daily use is a lower dose you take every day.
• Do not take tadalafil tablets more than one time each day.
• Take one tadalafil tablet every day at about the same time of day. You may attempt sexual activity at any time between doses.
• If you miss a dose, you may take it when you remember but do not take more than one dose per day.
• Some form of sexual stimulation is needed for an erection to happen with tadalafil tablets.
• Your healthcare provider may change your dose of tadalafil tablets depending on how you respond to the medicine, and on your health condition.
How Should I Take Tadalafil Tablets for Both ED and the Symptoms of BPH?
For both ED and the symptoms of BPH, tadalafil tablet is taken once daily.
• Do not take tadalafil tablet more than one time each day.
• Take one tadalafil tablet every day at about the same time of day. You may attempt sexual activity at any time between doses.
• If you miss a dose, you may take it when you remember but do not take more than one dose per day.
• Some form of sexual stimulation is needed for an erection to happen with tadalafil tablets.
What Should I Avoid While Taking Tadalafil Tablets?
• Do not use other ED medicines or ED treatments while taking tadalafil tablets.
• Do not drink too much alcohol when taking tadalafil tablets (for example, 5 glasses of wine or 5 shots of whiskey). Drinking too much alcohol can increase your chances of getting a headache or getting dizzy, increasing your heart rate, or lowering your blood pressure.
What Are The Possible Side Effects Of Tadalafil Tablets?
See "What Is The Most Important Information I Should Know About Tadalafil Tablets?"
The most common side effects with tadalafil tablets are:headache, indigestion, back pain, muscle aches, flushing, and stuffy or runny nose. These side effects usually go away after a few hours. Men who get back pain and muscle aches usually get it 12 to 24 hours after taking tadalafil tablets. Back pain and muscle aches usually go away within 2 days.
Call your healthcare provider if you get any side effect that bothers you or one that does not go away.
Uncommon side effects include:
An erection that won’t go away (priapism). If you get an erection that lasts more than 4 hours, get medical help right away. Priapism must be treated as soon as possible or lasting damage can happen to your penis, including the inability to have erections.
Color vision changes, such as seeing a blue tinge (shade) to objects or having difficulty telling the difference between the colors blue and green.
In rare instances, men taking PDE5 inhibitors (oral erectile dysfunction medicines, including tadalafil tablets) reported a sudden decrease or loss of vision in one or both eyes. It is uncertain whether PDE5 inhibitors directly cause the vision loss. If you experience sudden decrease or loss of vision, stop taking PDE5 inhibitors, including tadalafil tablets, and call a healthcare provider right away.
Sudden loss or decrease in hearing, sometimes with ringing in the ears and dizziness, has been rarely reported in people taking PDE5 inhibitors, including tadalafil tablets. It is not possible to determine whether these events are related directly to the PDE5 inhibitors, to other diseases or medications, to other factors, or to a combination of factors. If you experience these symptoms, stop taking tadalafil tablets and contact a healthcare provider right away.
These are not all the possible side effects of tadalafil tablets. For more information, ask your healthcare provider or pharmacist.
How Should I Store Tadalafil Tablets?
Store tadalafil tablets at room temperature between 20° to 25°C (68° to 77°F).
Keep tadalafil tablets and all medicines out of the reach of children.
General Information About Tadalafil Tablets:
Medicines are sometimes prescribed for conditions other than those described in patient information leaflets. Do not use tadalafil tablets for a condition for which it was not prescribed. Do not give tadalafil tablets to other people, even if they have the same symptoms that you have. It may harm them.
This is a summary of the most important information about tadalafil tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about tadalafil tablets that is written for health providers. For more information you can call 1-866-495-1995.
What Are The Ingredients In Tadalafil Tablets?
Active Ingredient: tadalafil USP
Inactive Ingredients: Colloidal silicon dioxide, copovidone, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyoxyl 40 hydrogenated castor oil, talc, titanium dioxide and triacetin. In addition, 2.5 mg contains FD&C blue #2/indigo carmine aluminum lake.
This Patient Information has been approved by the U.S. Food and Drug Administration
Rx only
*The brands listed are trademarks of their respective owners and are not trademarks of Evaric Pharmaceuticals Inc.
Revised Date: 11/24
Manufactured for:
XLCare Pharmaceuticals, Inc., 242 South Culver Street,
Suite 202 Lawrenceville, GA 30046.
Manufactured by:
Evaric Pharmaceuticals Inc.
155 Commerce Drive,
Hauppauge, New York 11788,
United States (USA). -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELTadalafil Tablets, USP 2.5 mg container label - Tadalafil Tablets, USP 5 mg container label - Tadalafil Tablets, USP 10 mg container label - Tadalafil Tablets, USP 20 mg container ...
Tadalafil Tablets, USP 2.5 mg container label
 Tadalafil Tablets, USP 5 mg container label
Tadalafil Tablets, USP 5 mg container label

Tadalafil Tablets, USP 10 mg container label

Tadalafil Tablets, USP 20 mg container label

Close -
INGREDIENTS AND APPEARANCEProduct Information
TADALAFIL tadalafil tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72865-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TADALAFIL (UNII: 742SXX0ICT) (TADALAFIL - UNII:742SXX0ICT) TADALAFIL 2.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE (UNII: D9C330MD8B) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) Product Characteristics Color blue Score no score Shape ROUND Size 6mm Flavor Imprint Code 122;E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72865-104-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209908 12/10/2024 TADALAFIL tadalafil tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72865-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TADALAFIL (UNII: 742SXX0ICT) (TADALAFIL - UNII:742SXX0ICT) TADALAFIL 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE (UNII: D9C330MD8B) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code 123;E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72865-106-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209908 12/10/2024 TADALAFIL tadalafil tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72865-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TADALAFIL (UNII: 742SXX0ICT) (TADALAFIL - UNII:742SXX0ICT) TADALAFIL 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE (UNII: D9C330MD8B) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) Product Characteristics Color white Score no score Shape CAPSULE Size 10mm Flavor Imprint Code 124;E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72865-107-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209908 12/10/2024 TADALAFIL tadalafil tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72865-108 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TADALAFIL (UNII: 742SXX0ICT) (TADALAFIL - UNII:742SXX0ICT) TADALAFIL 20 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE (UNII: D9C330MD8B) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) Product Characteristics Color white Score no score Shape CAPSULE Size 12mm Flavor Imprint Code 125;E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72865-108-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209908 12/10/2024 Labeler - XLCare Pharmaceuticals Inc. (080991142)
CloseEstablishment Name Address ID/FEI Business Operations Evaric Pharmaceuticals Inc 080470330 manufacture(72865-104, 72865-106, 72865-107, 72865-108)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
TADALAFIL- tadalafil tablet, film coated
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Dec 10, 2024 | 1 (current) | download |
RxNorm
TADALAFIL- tadalafil tablet, film coated
Under Review - Editing is pending for RxNorm. If in scope, these drugs will include RxNorm normal forms when editing is complete.
Get Label RSS Feed for this Drug
TADALAFIL- tadalafil tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=add6afb8-d38c-47a2-8c35-d5940667bb0c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
TADALAFIL- tadalafil tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 72865-104-30 |
| 2 | 72865-106-30 |
| 3 | 72865-107-30 |
| 4 | 72865-108-30 |




