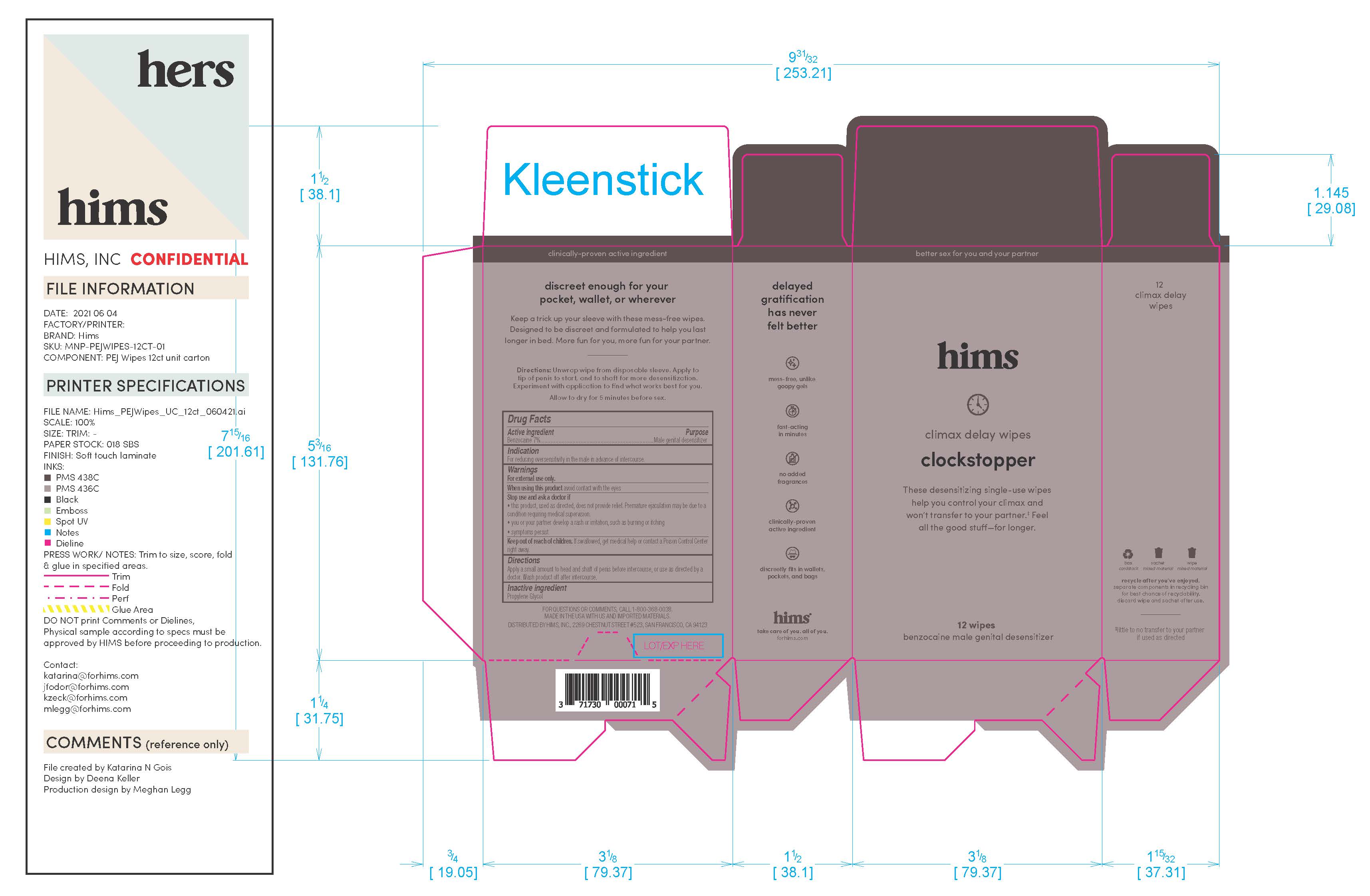
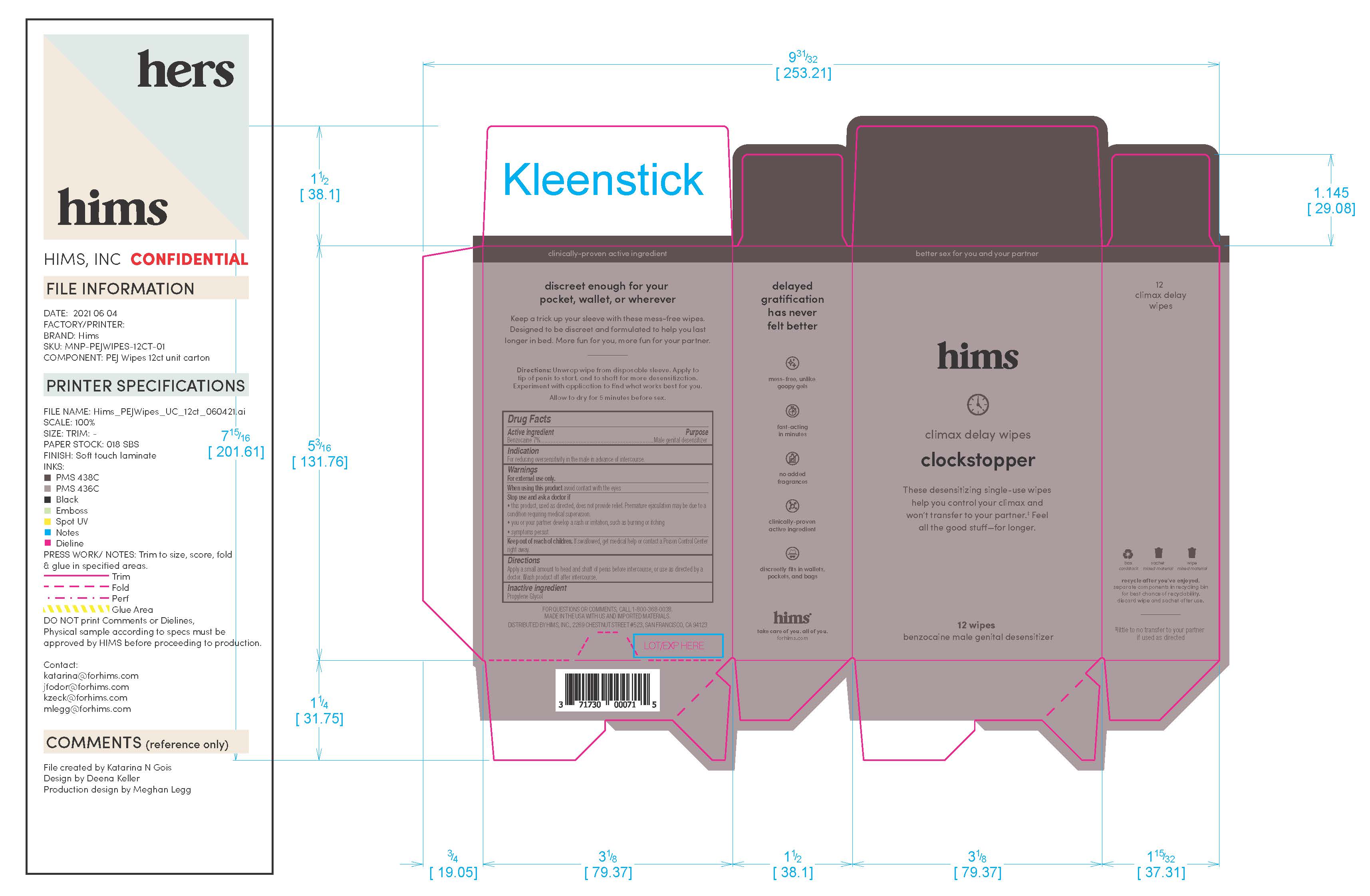
Label: HIMS DELAY WIPES- benzocaine wipes dressing
- NDC Code(s): 71730-009-03, 71730-009-12
- Packager: Hims Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Warnings
For external use only
When using this prouct avoid contact with the eyes
Stop and ask doctor if: the product, used as directed, does not provide relief. Premature ejaculation may be due to a condition requiring medical supervision. You or your partner develop a rash or irritation, such as burning or itching. If symptoms persist.If swallowed, get medical help or contact a Poison Control Center right away.
- Directions
- Indications
- Indications
- Active Ingredient
- Inactive Ingredient
- Keep out of reach of children
- Quesitons?
- Other Information
- Labeling
-
INGREDIENTS AND APPEARANCE
HIMS DELAY WIPES
benzocaine wipes dressingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71730-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 70 g in 1000 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71730-009-03 3 in 1 CARTON 06/14/2021 1 3 g in 1 POUCH; Type 0: Not a Combination Product 2 NDC:71730-009-12 12 in 1 CARTON 06/14/2021 2 3 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/14/2021 Labeler - Hims Inc. (080678637) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(71730-009)