Label: BACTINE MAX- lidocaine liquid
- NDC Code(s): 65197-832-03
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (w/w)
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- If you are allergic to any of the ingredients
- in or near the eyes
- over large areas of the body
- longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
- Questions?
- Distributed by:
-
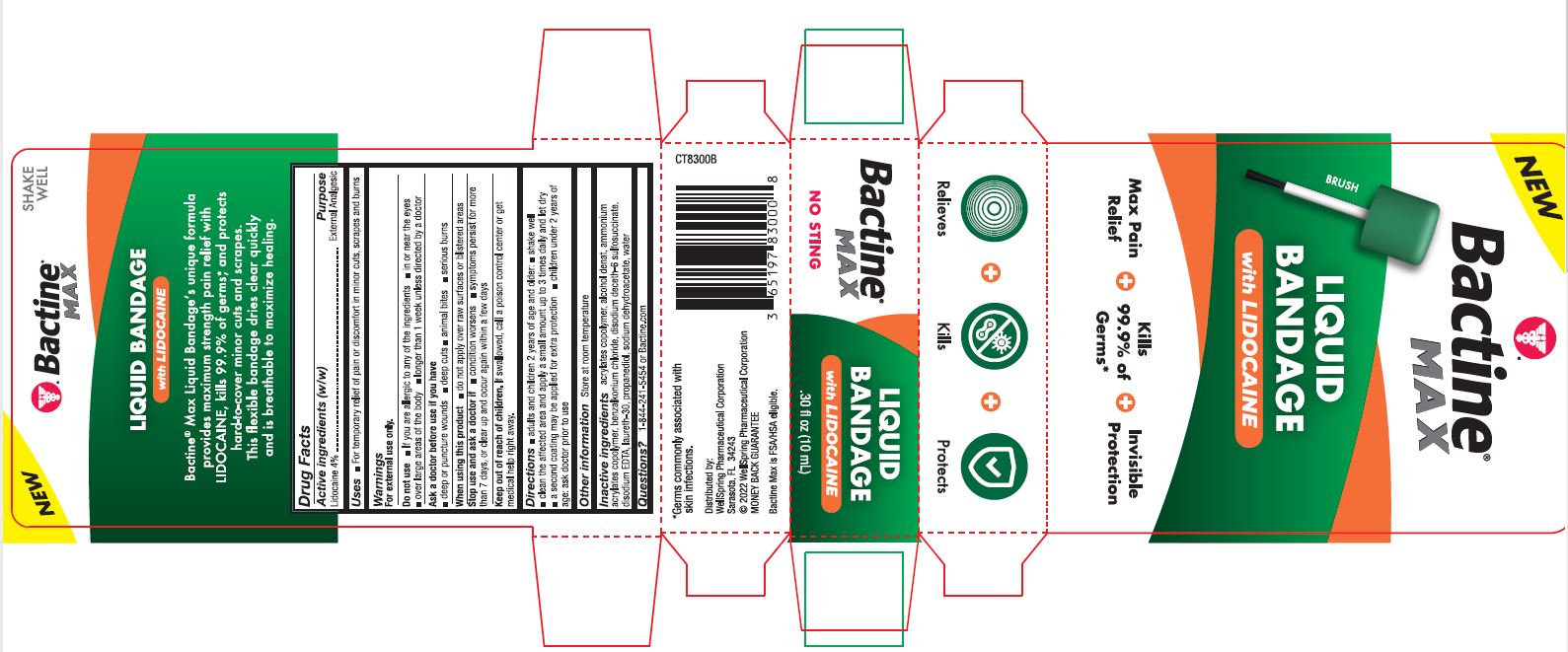
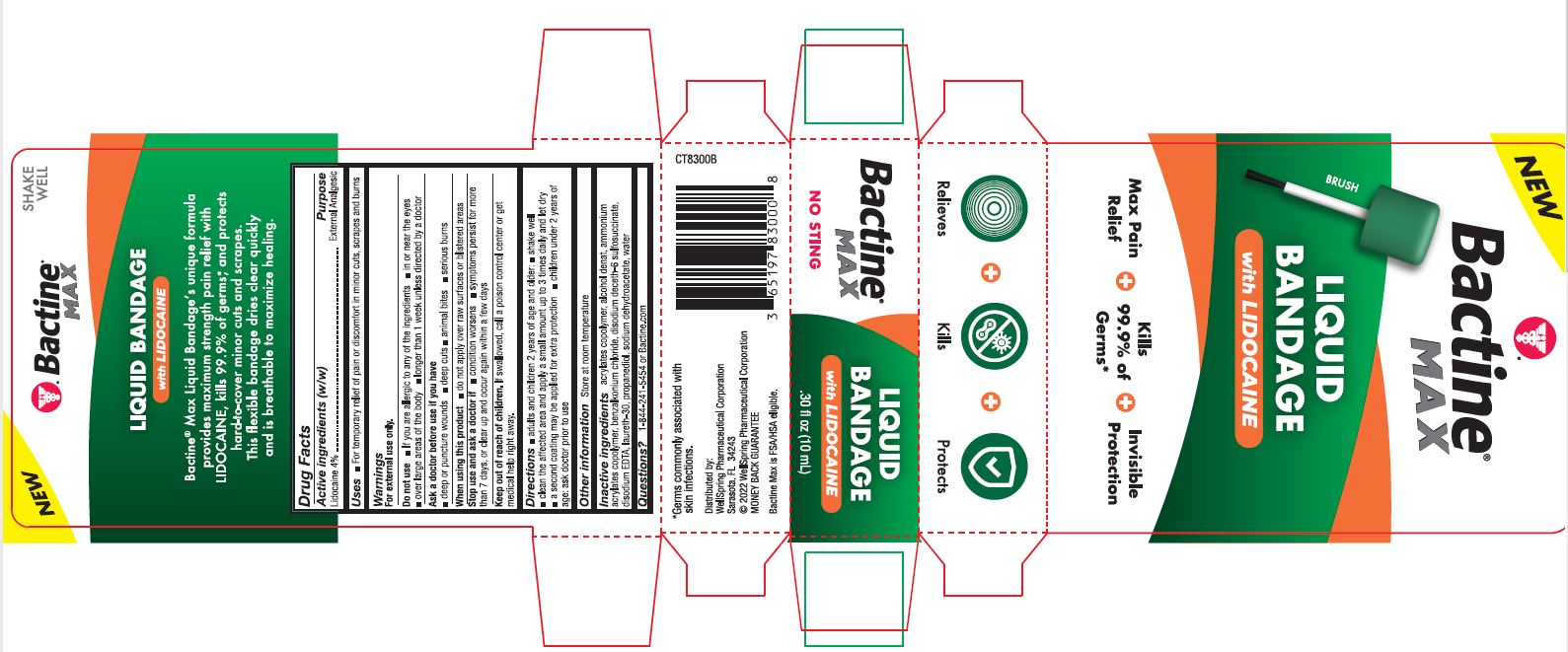
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
New
Bactine Max
Liquid Bandage with Lidocaine
Max Pain Relief
Kills 99% of Germs
Invisible Protection
Relieves Kills Protects
NO STING
SHAKE WELL
Bactine Max Liquid Bandage with Lidocaine
Back Panel
New
Bactine Max
Liquid Bandage with Lidocaine
SHAKE WELL
Bactine Max Liquid Bandageʼs unique formula provides maximum strength pain relief with LIDOCAINE, kills 99.9% of germs*, and protects hard-to-cover minor cuts and scrapes. This flexible bandage dries clear quickly and is breathable to maximize healing.
-
INGREDIENTS AND APPEARANCE
BACTINE MAX
lidocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-832 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 mL Inactive Ingredients Ingredient Name Strength BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) ALCOHOL (UNII: 3K9958V90M) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPANEDIOL (UNII: 5965N8W85T) LAURETH-30 (UNII: W9D845551A) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-832-03 1 in 1 BOX 04/01/2022 1 10 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/01/2022 Labeler - WellSpring Pharmaceutical Corporation (110999054)