Label: BUSPIRONE HYDROCHLORIDE tablet
- NDC Code(s): 71335-2510-1, 71335-2510-2, 71335-2510-3, 71335-2510-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0093-5200
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
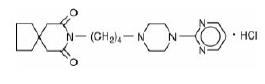
DESCRIPTIONBuspirone hydrochloride tablets, USP are an antianxiety agent that is not chemically or pharmacologically related to the benzodiazepines, barbiturates, or other sedative/anxiolytic ...
-
CLINICAL PHARMACOLOGYThe mechanism of action of buspirone is unknown. Buspirone differs from typical benzodiazepine anxiolytics in that it does not exert anticonvulsant or muscle relaxant effects. It also lacks the ...
-
INDICATIONS AND USAGEBuspirone hydrochloride tablets are indicated for the management of anxiety disorders or the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday ...
-
CONTRAINDICATIONSBuspirone hydrochloride tablets are contraindicated in patients hypersensitive to buspirone hydrochloride. The use of monoamine oxidase inhibitors (MAOIs) intended to treat depression with ...
-
WARNINGSThe administration of buspirone hydrochloride tablets to a patient taking a monoamine oxidase inhibitor (MAOI) may pose a hazard. There have been reports of the occurrence of elevated blood ...
-
PRECAUTIONSGeneral - Interference with Cognitive and Motor Performance - Studies indicate that buspirone hydrochloride tablets is less sedating than other anxiolytics and that it does not produce ...
-
ADVERSE REACTIONS(See also PRECAUTIONS.) Commonly Observed - The more commonly observed untoward events associated with the use of buspirone hydrochloride tablets not seen at an equivalent incidence among ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance Class - Buspirone hydrochloride is not a controlled substance. Physical and Psychological Dependence - In human and animal studies, buspirone has shown no potential for ...
-
OVERDOSAGESigns and Symptoms - In clinical pharmacology trials, doses as high as 375 mg/day were administered to healthy male volunteers. As this dose was approached, the following symptoms were observed ...
-
DOSAGE AND ADMINISTRATIONThe recommended initial dose is 15 mg daily (7.5 mg b.i.d.). To achieve an optimal therapeutic response, at intervals of 2 to 3 days the dosage may be increased 5 mg per day, as needed. The ...
-
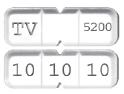
HOW SUPPLIEDBusPIRone Hydrochloride Tablets USP, 30 mg are available as white to off-white, rectangular tablets that can either be bisected or trisected, debossed “TV” and “5200” on bisect segments, and ...
-
REFERENCESAmerican Psychiatric Association, Ed.: Diagnostic and Statistical Manual of Mental Disorders—III, American Psychiatric Association, May 1980. Manufactured In Croatia By: Pliva Hrvatska ...
-
PATIENT PACKAGE INSERTDispense with Patient Package Insert available at: www.tevausa.com/PatientPI - PATIENT INFORMATION - BusPIRone Hydrochloride Tablets, USP - Rx only - HOW TO USE: BusPIRone Hydrochloride Tablets ...
-
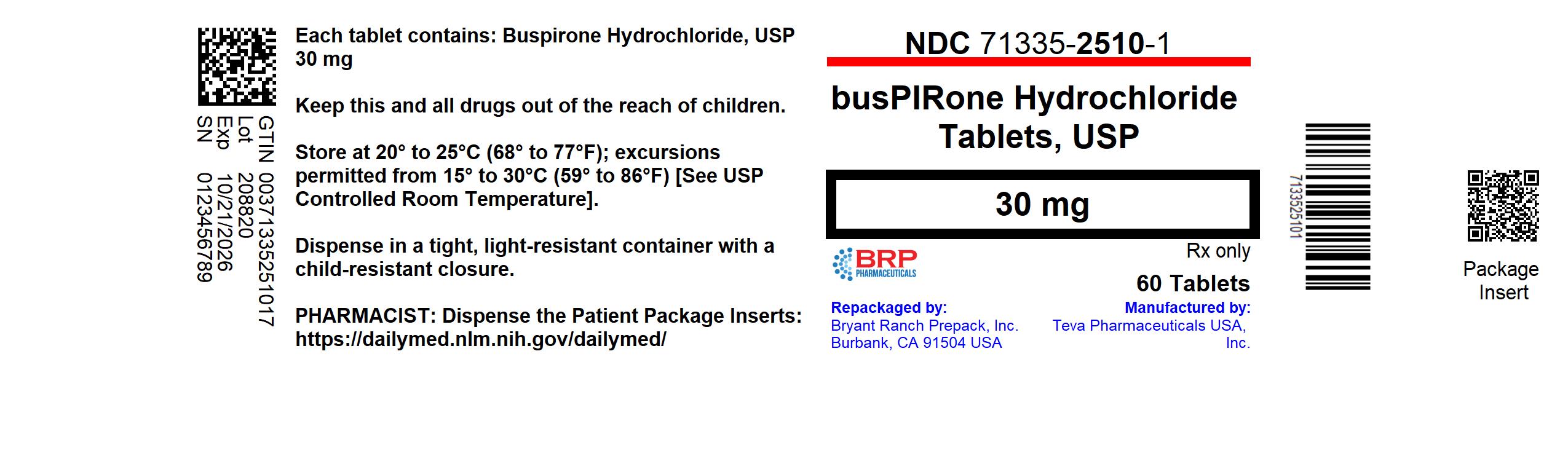
PRINCIPAL DISPLAY PANELBuspirone Hcl 30mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information