Label: AMIODARONE HYDROCHLORIDE injection, solution
- NDC Code(s): 14335-430-10, 14335-431-10, 14335-432-10
- Packager: Hainan Poly Pharm. Co., Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMIODARONE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for AMIODARONE HYDROCHLORIDE INJECTION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAmiodarone injection is indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in ...
-
2 DOSAGE AND ADMINISTRATIONAmiodarone shows considerable interindividual variation in response. Although a starting dose adequate to suppress life-threatening arrhythmias is needed, close monitoring with adjustment of dose ...
-
3 DOSAGE FORMS AND STRENGTHSInjection, 50 mg/mL
-
4 CONTRAINDICATIONSAmiodarone is contraindicated in patients with: • Known hypersensitivity to any of the components of Amiodarone Hydrochloride Injection, USP, including iodine. Hypersensitivity reactions may ...
-
5 WARNINGS AND PRECAUTIONSAmiodarone should be administered only by physicians who are experienced in the treatment of life-threatening arrhythmias, who are thoroughly familiar with the risks and benefits of amiodarone ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: • Hypotension [see Warnings and Precautions (5.1)] • Hepatic injury [see Warnings and Precautions (5.3)] • Rhythm ...
-
7 DRUG INTERACTIONS7.1 Pharmacodynamic Interactions - Drugs Prolonging the QT Interval - Co-administration of drugs prolonging the QT interval (such as class I and III antiarrhythmics, lithium, certain ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects - Amiodarone and desethylamiodarone cross the placenta. Reported risks include: • neonatal bradycardia, QT prolongation, and periodic ventricular ...
-
10 OVERDOSAGEThere have been cases, some fatal, of amiodarone overdose. Effects of an inadvertent overdose of intravenous amiodarone include hypotension, cardiogenic shock, bradycardia, AV block, and ...
-
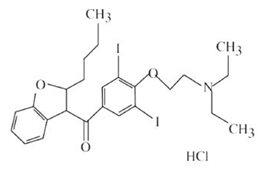
11 DESCRIPTIONAmiodarone Hydrochloride Injection, USP contains amiodarone hydrochloride, USP (C - 25H - 29I - 2NO - 3•HCl), a class III antiarrhythmic drug. Amiodarone hydrochloride is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Amiodarone is generally considered a class III antiarrhythmic drug, but it possesses electrophysiologic characteristics of all four Vaughan Williams classes. Like class ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity studies were conducted with intravenous administration of amiodarone. However, oral amiodarone caused a ...
-
14 CLINICAL STUDIESApart from studies in patients with VT or VF, described below, there are two other studies of amiodarone showing an antiarrhythmic effect before significant levels of DEA could have accumulated. A ...
-
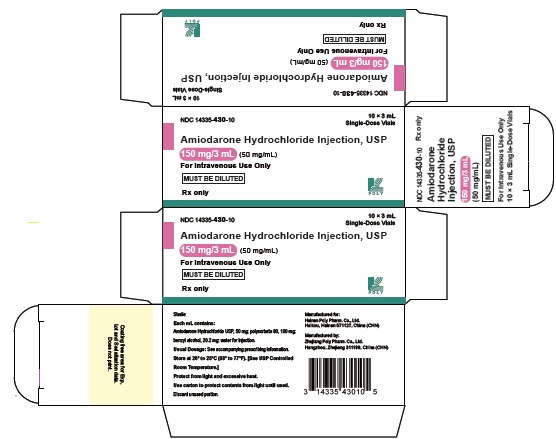
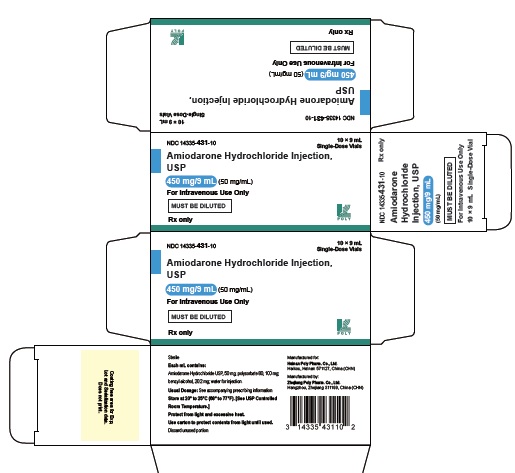
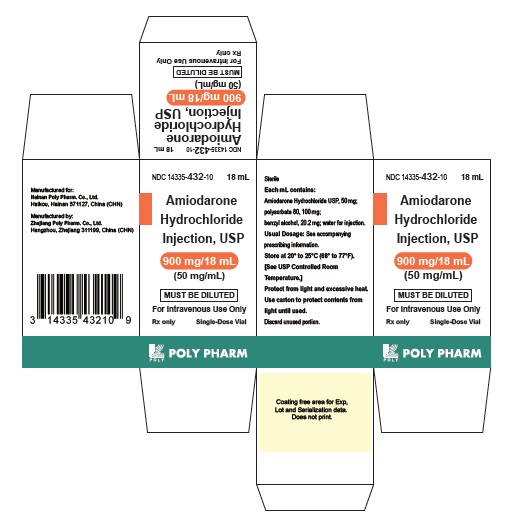
16 HOW SUPPLIED/STORAGE AND HANDLINGAmiodarone Hydrochloride Injection USP, 50 mg/mL, is available in single-use glass vials packaged in individual cartons as follows: 150 mg/3 mL - single-dose vial - NDC 14335-430-10 ...
-
17 PATIENT COUNSELING INFORMATIONAmiodarone has the potential to cause serious side effects that limit its use to life-threatening and hemodynamically unstable cardiac arrhythmias. Advise female patients to discontinue nursing ...
-
PRINCIPAL DISPLAY PANEL - 50 mg/mLNDC 67457-153-03 - AmiodaroneHydrochloride - Injection, USP - 150 mg/3 mL (50 mg/mL) For Intravenous Use Only - MUST BE DILUTED - Rx only 10 x 3 mL Single-Dose Vials - Sterile - Each mL ...
-
PRINCIPAL DISPLAY PANEL - 50 mg/mLNDC 14335-431-10 - AmiodaroneHydrochloride - Injection, USP - 450 mg/9 mL (50 mg/mL) For Intravenous Use Only - MUST BE DILUTED - Rx only 10 x 9 mL Single-Dose Vials - Sterile - Each mL ...
-
PRINCIPAL DISPLAY PANEL - 50 mg/mLNDC 14335-432-10 - AmiodaroneHydrochloride - Injection, USP - 900 mg/18 mL (50 mg/mL) For Intravenous Use Only - MUST BE DILUTED - Rx only 1 x 18 mL Single-Dose Vials - Sterile - Each mL ...
-
INGREDIENTS AND APPEARANCEProduct Information