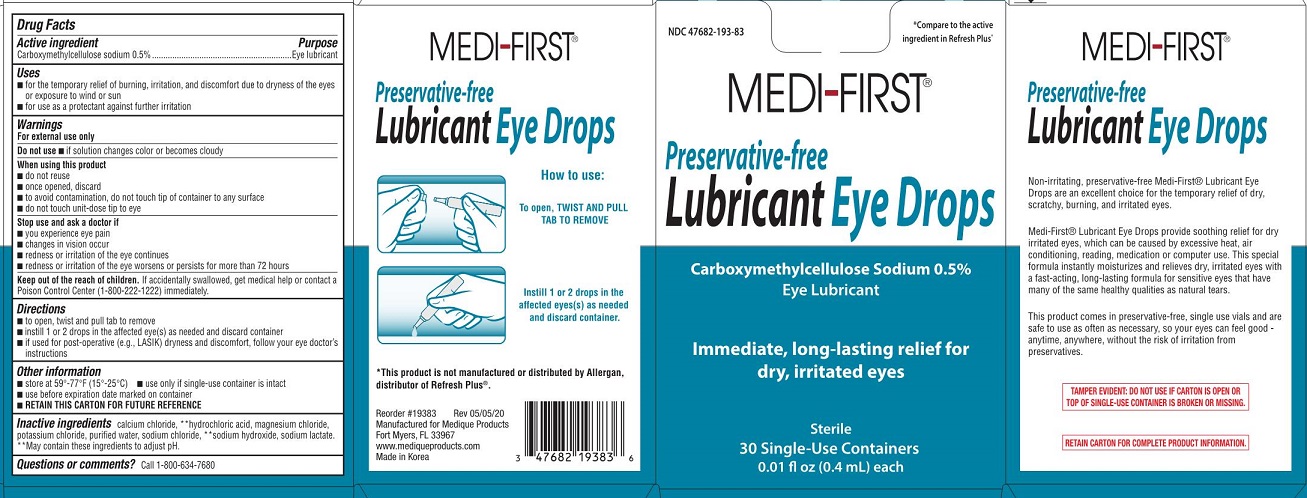
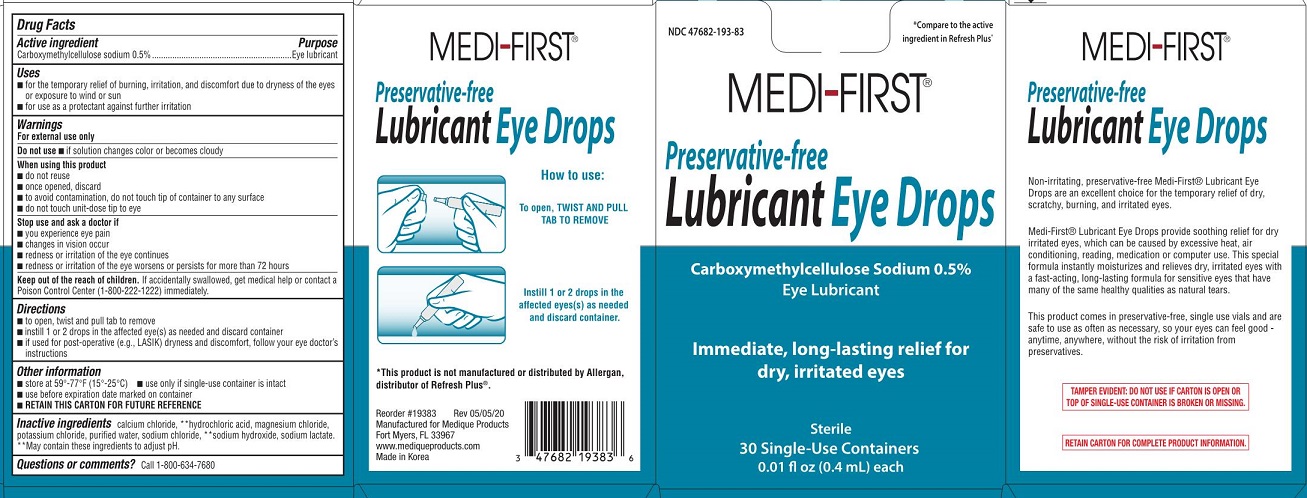
Label: MEDI-FIRST LUBRICANT EYE DROPS- carboxymethylcellulose sodium solution
- NDC Code(s): 47682-193-83
- Packager: Unifirst First Aid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active Ingredient
Carboxymethylcellulose sodium 0.5%
-
Purpose
Eye lubricant
-
Uses
for the temporary relief of burning, irritation, and discomfort due to dryness of the eyes or exposure to wind or sun - for use a protectant against further irritation
-
Warnings
For external use only - Do not use - if solution changes color or becomes cloudy - When using this product - do not reuse - once opened, discard - to avoid contamination, do not touch tip of ...
-
KEEP OUT OF REACH OF CHILDRENKeep out od reach of children. If accidentally swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediately.
-
Directions
to open, twist and pull tab to remove - instill 1 or 2 drops in the affected eye(s) as needed and discard container - if used for post-operative (e.g.,LASIK) dryness and discomfort, follow your eye ...
-
Other information
store at 59º-77ºF (15º-25ºC) use only if single use container is intact - use before expiration date marked on container - RETAIN THIS CARTON FOR FUTURE REFERENCE
-
Inactive ingredients
calcium chloride, **hydrochloric acid, magmesium chloride, potassium chloride, purified water, sodium chloride, **sodium hydroxide, sodium lactate - ** May contain these ingredients to adjust ...
-
Questions or comments?
Call 1-800-634-7680
-
Medi-First Lubricant Eye Drops LabelNDC 47682-193-83 - *Compare to the active ingredient in Refresh Plus® Medi-First® Preservative-free - Lubricant Eye Drops - Carboxymethylcellulose Sodium 0.5% Eye Lubricant - Immediate, long-lasting ...
-
INGREDIENTS AND APPEARANCEProduct Information