Label: RYONCIL- remestemcel-l-rknd kit
- NDC Code(s): 73648-111-01, 73648-112-02, 73648-113-03, 73648-114-01, view more
- Packager: Mesoblast
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RYONCIL® safely and effectively. See full prescribing information for RYONCIL®. RYONCIL® (remestemcel-L-rknd) suspension for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE RYONCIL is indicated for the treatment of steroid refractory acute graft versus host disease (SR-aGvHD) in pediatric patients 2 months of age and older.
-
2 DOSAGE AND ADMINISTRATION For intravenous use only. 2.1 Recommended Dosage - The recommended dosage of RYONCIL is 2 × 106 mesenchymal stromal cells (MSC)/kg body weight per intravenous infusion given twice a week for ...
-
3 DOSAGE FORMS AND STRENGTHSRYONCIL is available as a cell suspension for intravenous infusion in a target concentration of 6.68 X 106 MSCs per mL in 3.8 mL at cryopreservation contained in a 6 mL cryovial. Each 6 mL ...
-
4 CONTRAINDICATIONS Do not use RYONCIL in patients with known hypersensitivity to dimethyl sulfoxide (DMSO) or porcine and bovine proteins.
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity and Acute Infusion Reactions - Hypersensitivity reactions including acute infusion reactions have occurred with RYONCIL administration [see Adverse Reactions (6.1)]. Serious ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no available data for RYONCIL use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with RYONCIL to assess ...
-
11 DESCRIPTIONRYONCIL is provided as a frozen cell suspension in a cryogenic vial. The active ingredient in RYONCIL is comprised of culture-expanded mesenchymal stromal cells (MSCs) isolated from the bone ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action for RYONCIL is not clear but may be related to immunomodulatory effects. Data from in vitro studies demonstrate that MSCs inhibit T cell ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No animal studies have been performed to evaluate the effects of RYONCIL on carcinogenesis, mutagenesis or impairment of ...
-
14 CLINICAL STUDIESThe efficacy of RYONCIL was evaluated in a multicenter, prospective, single-arm study (MSB-GVHD 001; NCT02336230). The study enrolled pediatric patients with SR-GvHD Grade B to D (excluding Grade ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRYONCIL is supplied as a sterile, cryopreserved cell suspension of ex-vivo culture-expanded allogeneic bone marrow-derived mesenchymal stromal cells (MSC) in vials. RYONCIL is provided as a ...
-
17 PATIENT COUNSELING INFORMATIONDiscuss the following with patients and/or caregivers. Hypersensitivity and Acute infusion reactions: Inform patients and/or caregivers that hypersensitivity and acute infusion reactions due to ...
-
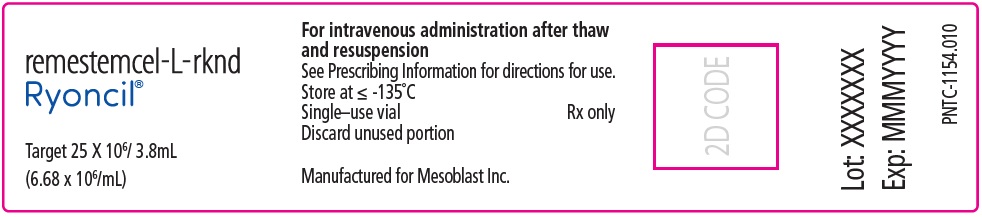
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL remestemcel-L-rknd - Ryoncil® Target 25 X 106/ 3.8mL - (6.68 x 106/mL) For intravenous administration after thaw - and resuspension - See Prescribing Information for directions ...
-
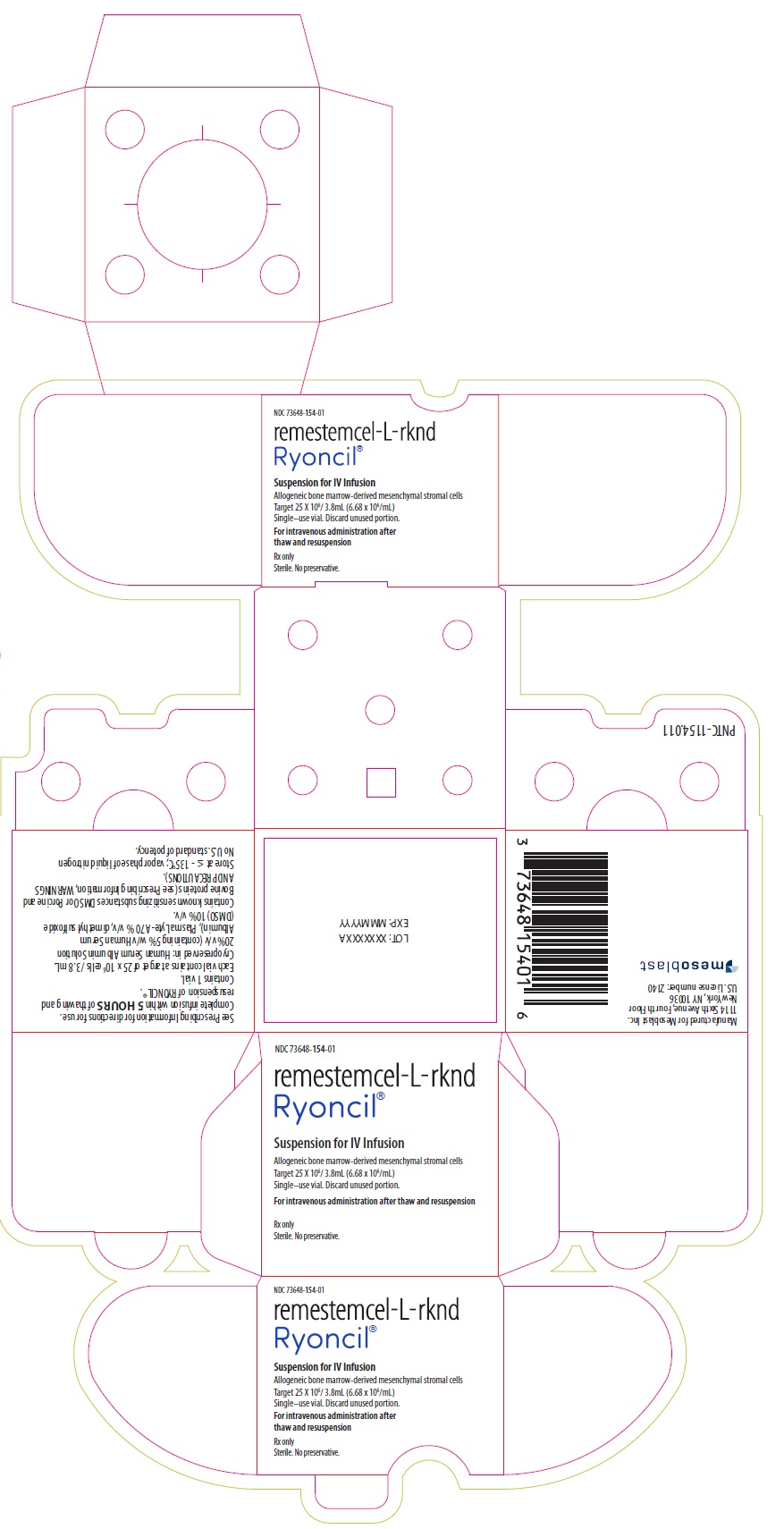
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-154-01 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL ...
-
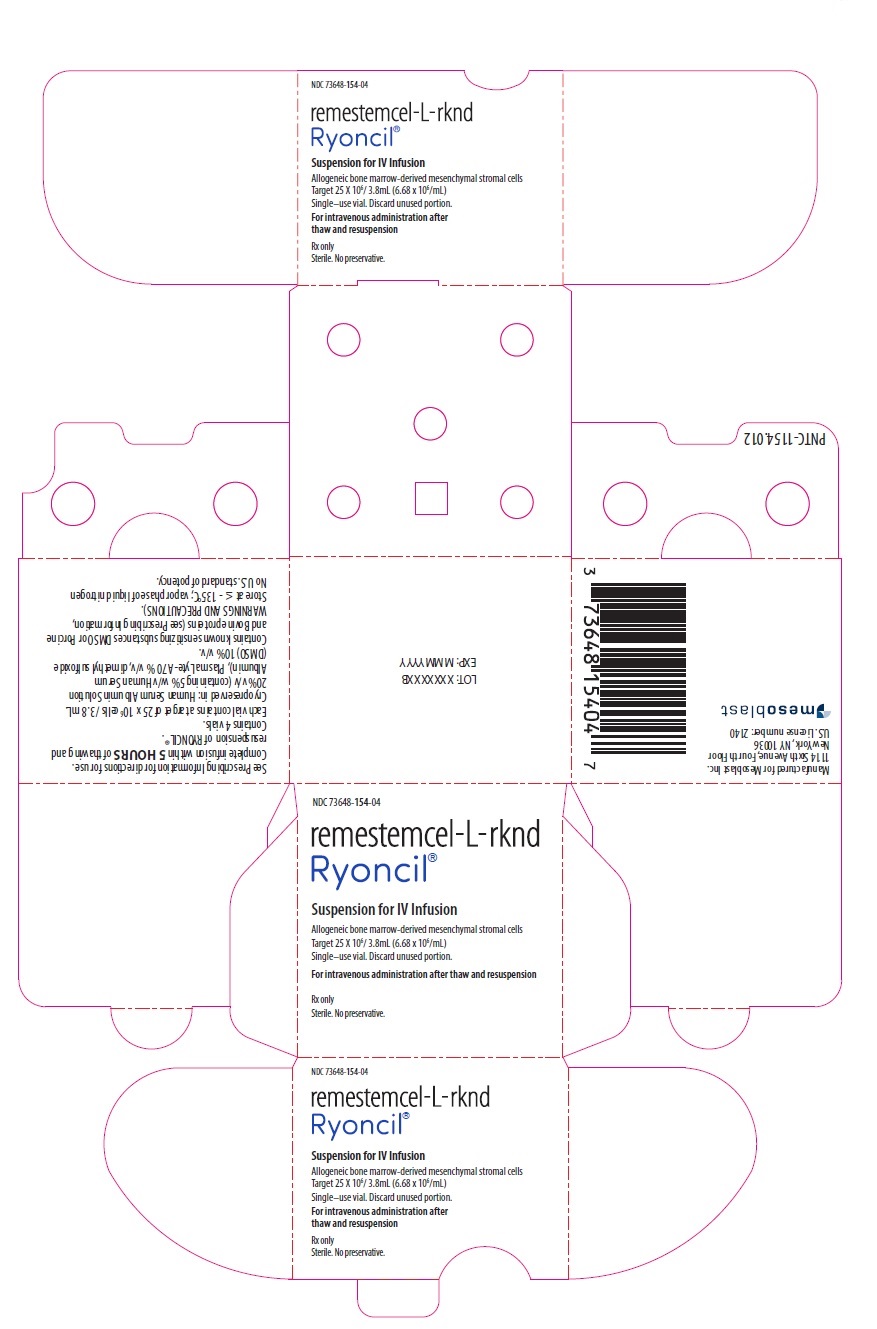
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-154-04 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-111-01 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-112-02 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-113-03 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-114-01 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-115-02 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-116-03 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-117-04 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 73648-118-02 - remestemcel-L-rknd - Ryoncil® Suspension for IV Infusion - Allogeneic bone marrow-derived mesenchymal stromal cells - Target 25 X 106/ 3.8mL (6.68 x 106/mL) Kit for ...
-
INGREDIENTS AND APPEARANCEProduct Information