Label: HYMPAVZI- marstacimab-hncq injection, solution
- NDC Code(s): 0069-2151-01
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HYMPAVZI safely and effectively. See full prescribing information for HYMPAVZI. HYMPAVZI (marstacimab-hncq) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE HYMPAVZI is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with: • hemophilia A (congenital ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - For subcutaneous use only. The recommended dosage of HYMPAVZI for adult and pediatric patients 12 years of age and older is as follows: Loading Dose - 300 mg (two ...
-
3 DOSAGE FORMS AND STRENGTHS HYMPAVZI (marstacimab‑hncq) is a clear and colorless to light yellow solution available as: Prefilled Syringe - • Injection: 150 mg/mL in a single-dose prefilled syringe - Prefilled ...
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Thromboembolic Events - HYMPAVZI is a tissue factor pathway inhibitor (TFPI) antagonist, and may increase the risk of thromboembolic complications. HYMPAVZI has not been studied in ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Thromboembolic Events [see Warnings and Precautions (5.1)] • Hypersensitivity [see Warnings and ...
-
7 DRUG INTERACTIONS Partial Thromboplastin Time (aPTT) and Prothrombin Time (PT) No clinically significant differences in standard measures of coagulation including activated partial thromboplastin time (aPTT) and ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on its mechanism of action, HYMPAVZI may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available ...
-
11 DESCRIPTION Marstacimab‑hncq is a tissue factor pathway inhibitor (TFPI) antagonist, human monoclonal immunoglobulin G Type 1 (IgG1) antibody. Marstacimab‑hncq is produced by Chinese hamster ovary (CHO) cells ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Marstacimab‑hncq is a human monoclonal IgG1 antibody directed against the Kunitz domain 2 (K2) of TFPI to neutralize TFPI activity and enhance coagulation. TFPI is ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted to assess marstacimab‑hncq for the potential for carcinogenicity or mutagenicity. Marstacimab‑hncq ...
-
14 CLINICAL STUDIES 14.1 Hemophilia A without FVIII Inhibitors or Hemophilia B without FIX Inhibitors - The efficacy of HYMPAVZI was established in 116 adult and pediatric patients (aged 12 years and older and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - HYMPAVZI (marstacimab‑hncq) injection is a sterile, preservative-free, clear and colorless to light yellow solution available as a 150 mg/mL single-dose prefilled syringe ...
-
17 PATIENT COUNSELING INFORMATION • Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use). • Ensure that patients and caregivers who will administer HYMPAVZI ...
-
Patient Package Insert This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2024 - PATIENT INFORMATION - HYMPAVZI ...
-
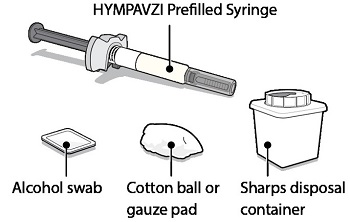
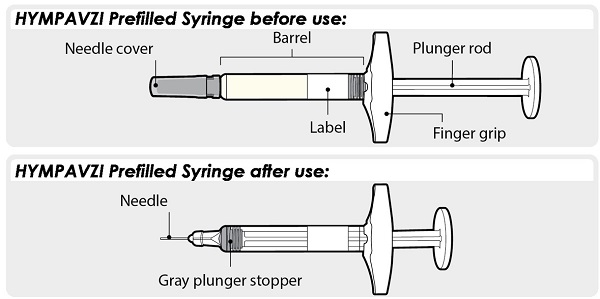
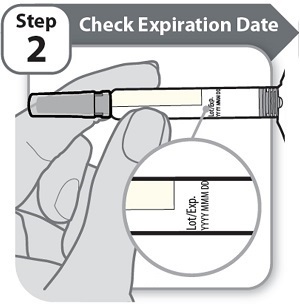
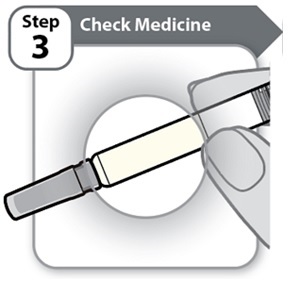
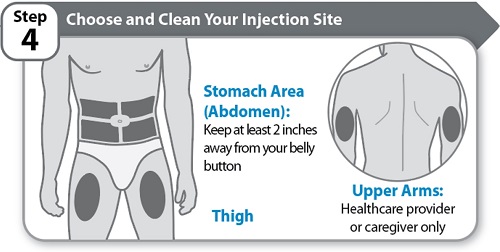
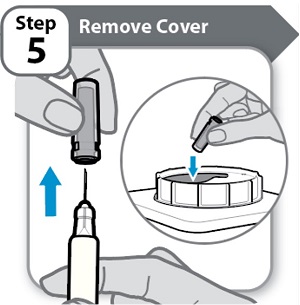
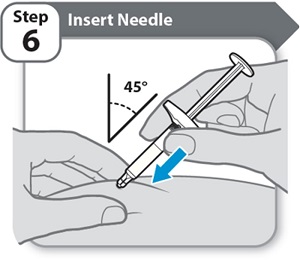
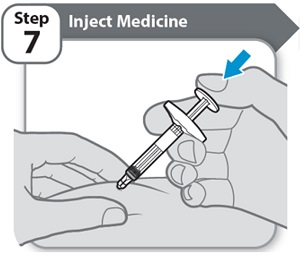
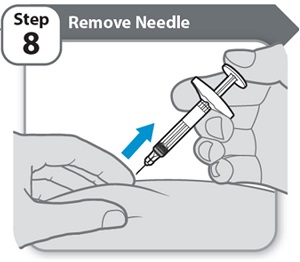
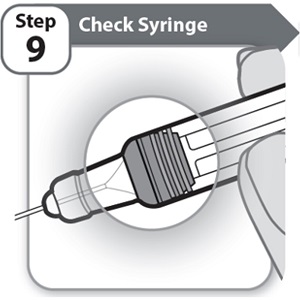
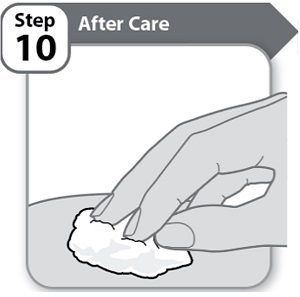
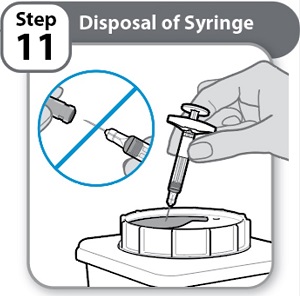
Instructions for Use INSTRUCTIONS FOR USE - HYMPAVZI™ (him-PAV-zee) (marstacimab-hncq) injection, for subcutaneous use - single-dose prefilled syringe - This Instructions for Use contains information on how to inject ...
-
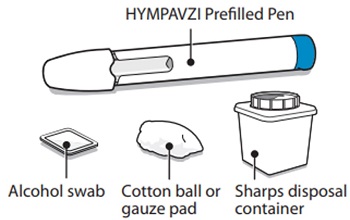
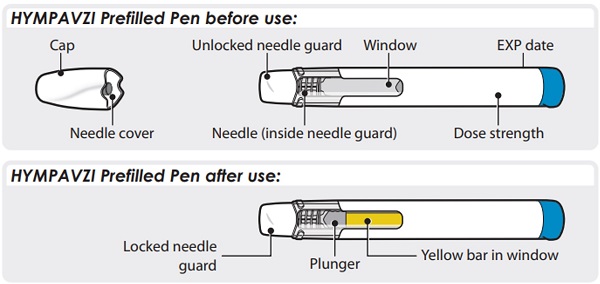
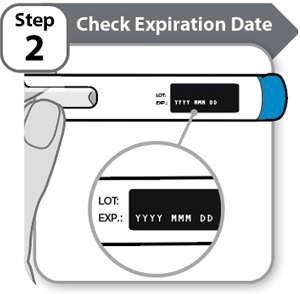
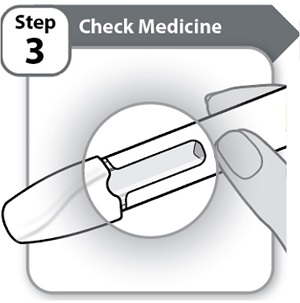
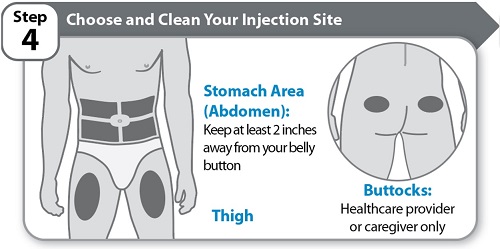
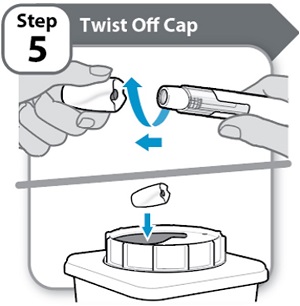
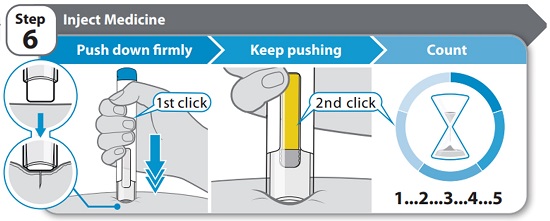
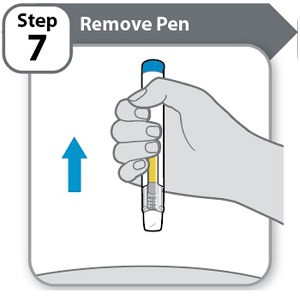
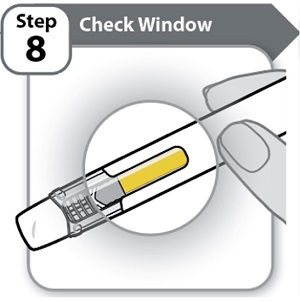
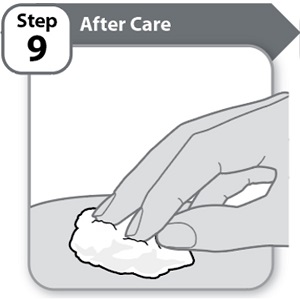
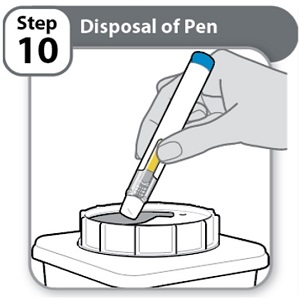
Instructions for Use INSTRUCTIONS FOR USE - HYMPAVZI™ (him-PAV-zee) (marstacimab-hncq) injection, for subcutaneous use - single-dose prefilled pen - This Instructions for Use contains information on how to inject ...
-
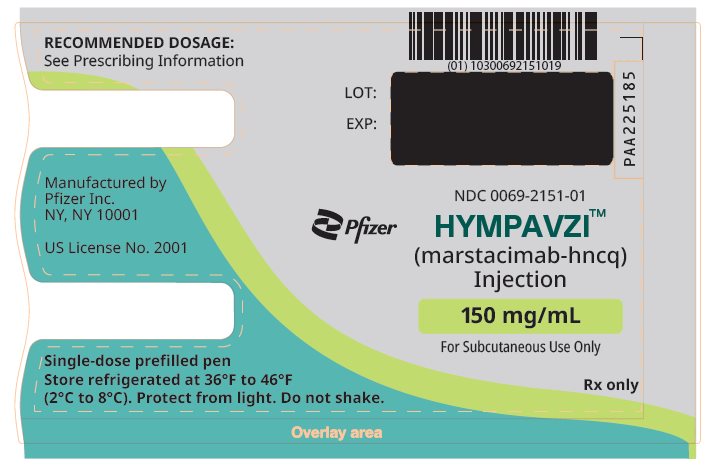
PRINCIPAL DISPLAY PANEL – 150 mg/mL Prefilled Pen NDC 0069-2151-01 - Pfizer - HYMPAVZITM - (marstacimab-hncq) Injection - 150 mg/mL - For Subcutaneous Use Only - Rx only
-
PRINCIPAL DISPLAY PANEL – 150 mg/mL Prefilled Pen Carton NDC 0069-2151-01 - 1 single-dose prefilled pen - Pfizer - HYMPAVZI TM - (marstacimab-hncq) Injection - 150 mg/mL - For Subcutaneous Use Only - READ ENCLOSED INSTRUCTIONS BEFORE USE - This carton contains: 1 ...
-
INGREDIENTS AND APPEARANCEProduct Information