Label: HYDRALAZINE HYDROCHLORIDE tablet
- NDC Code(s): 55154-2140-0, 55154-2141-0
- Packager: Cardinal Health 107, LLC
- This is a repackaged label.
- Source NDC Code(s): 60687-822, 60687-833
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
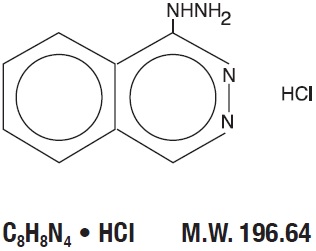
DESCRIPTIONHydrALAZINE hydrochloride, USP, is an antihypertensive, for oral administration. Its chemical name is 1-hydrazinophthalazine monohydrochloride, and its structural formula is: HydrALAZINE ...
-
CLINICAL PHARMACOLOGYAlthough the precise mechanism of action of hydrALAZINE is not fully understood, the major effects are on the cardiovascular system. HydrALAZINE apparently lowers blood pressure by exerting a ...
-
INDICATIONS AND USAGEEssential hypertension, alone or as an adjunct.
-
CONTRAINDICATIONSHypersensitivity to hydrALAZINE; coronary artery disease; mitral valvular rheumatic heart disease.
-
WARNINGSIn a few patients hydrALAZINE may produce a clinical picture simulating systemic lupus erythematosus including glomerulonephritis. In such patients hydrALAZINE should be discontinued unless the ...
-
PRECAUTIONSGeneral - Myocardial stimulation produced by hydrALAZINE can cause anginal attacks and ECG changes of myocardial ischemia. The drug has been implicated in the production of myocardial infarction ...
-
ADVERSE REACTIONSAdverse reactions with hydrALAZINE are usually reversible when dosage is reduced. However, in some cases it may be necessary to discontinue the drug. The following adverse reactions have been ...
-
OVERDOSAGEAcute Toxicity: No deaths due to acute poisoning have been reported. Highest known dose survived: adults, 10 g orally. Oral LD 50 in rats: 173 and 187 mg/kg. Signs and Symptoms - Signs and ...
-
DOSAGE AND ADMINISTRATIONInitiate therapy in gradually increasing dosages; adjust according to individual response. Start with 10 mg four times daily for the first 2 to 4 days, increase to 25 mg four times daily for the ...
-
HOW SUPPLIEDHydrALAZINE Hydrochloride Tablets, USP are available as: 25 mg – Round, peach, core tablet, debossed EP over 102 on one side and plain on the reverse side. Overbagged with 10 tablets per bag, NDC ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose blisters contain drug product from Avet Pharmaceuticals Inc. Distributed by: American Health Packaging - Columbus, OH 43217 Distributed By: Cardinal ...
-
Package/Label Display Panel NDC 55154-2141-0 - HydrALAZINE HYDROCHLORIDE - TABLETS, USP - 25 mg - 10 TABLETS
-
Package/Label Display Panel NDC 55154-2140-0 - HydrALAZINE HYDROCHLORIDE - TABLETS, USP - 50 mg - 10 TABLETS
-
INGREDIENTS AND APPEARANCEProduct Information