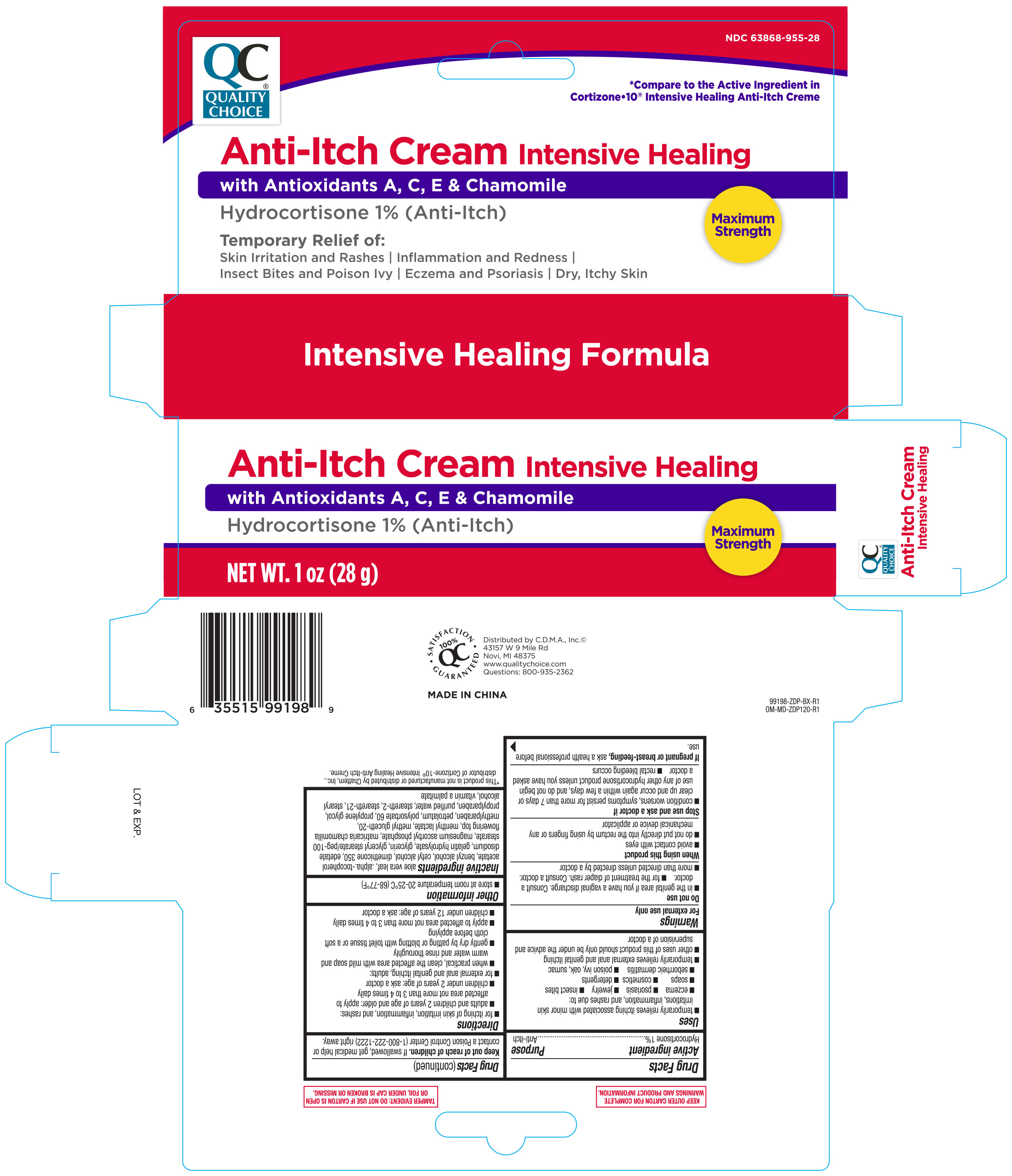
Label: QUALITY CHOICE ANTI ITCH- hydrocortisone cream
- NDC Code(s): 63868-955-28
- Packager: Chain Drug Marketing Association Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
PURPOSE
Uses
- temporarily relieves itching associated with minor skin irritation, inflammation, and rashes due to:
- eczema psoriasis
- jewelry
- insect bites
- soaps
- cosmetics
- detergents
- seborrheic dermatitis
- poison ivy, oak, sumac
- temporarily relieves external anal and genital itching
- other uses of this product should only be under the advice and supervision of a doctor
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Directions
- for itching of skin irritation, inflammation, and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- for external anal and genital itching, adults:
- when practical, clean the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
aloe vera leaf, .alpha.-tocopherol acetate, benzyl alcohol, cetyl alcohol, dimethicone 350, edetate disodium, gelatin hydrolysate, glycerin, glyceryl stearate/peg-100 stearate, magnesium ascorbyl, phosphate, matricaria chamomilla, flowering top, menthyl lactate,methyl gluceth-20, methylparaben, petrolatum,polysorbate 60, propylene glycol, propylparaben, purified water, steareth-2, steareth-21, stearyl alcohol, vitamin a palmitate
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE ANTI ITCH
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-955 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PETROLATUM (UNII: 4T6H12BN9U) BENZYL ALCOHOL (UNII: LKG8494WBH) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) STEARETH-21 (UNII: 53J3F32P58) POLYSORBATE 60 (UNII: CAL22UVI4M) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL STEARATE/PEG-100 STEARATE (UNII: RD25J5V947) MATRICARIA CHAMOMILLA FLOWERING TOP (UNII: 3VNC7T6Z02) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) METHYL GLUCETH-20 (UNII: J3QD0LD11P) METHYLPARABEN (UNII: A2I8C7HI9T) DIMETHICONE 350 (UNII: 2Y53S6ATLU) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) PROPYLPARABEN (UNII: Z8IX2SC1OH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARETH-2 (UNII: V56DFE46J5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-955-28 1 in 1 CARTON 12/10/2019 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/07/2019 Labeler - Chain Drug Marketing Association Inc (011920774)