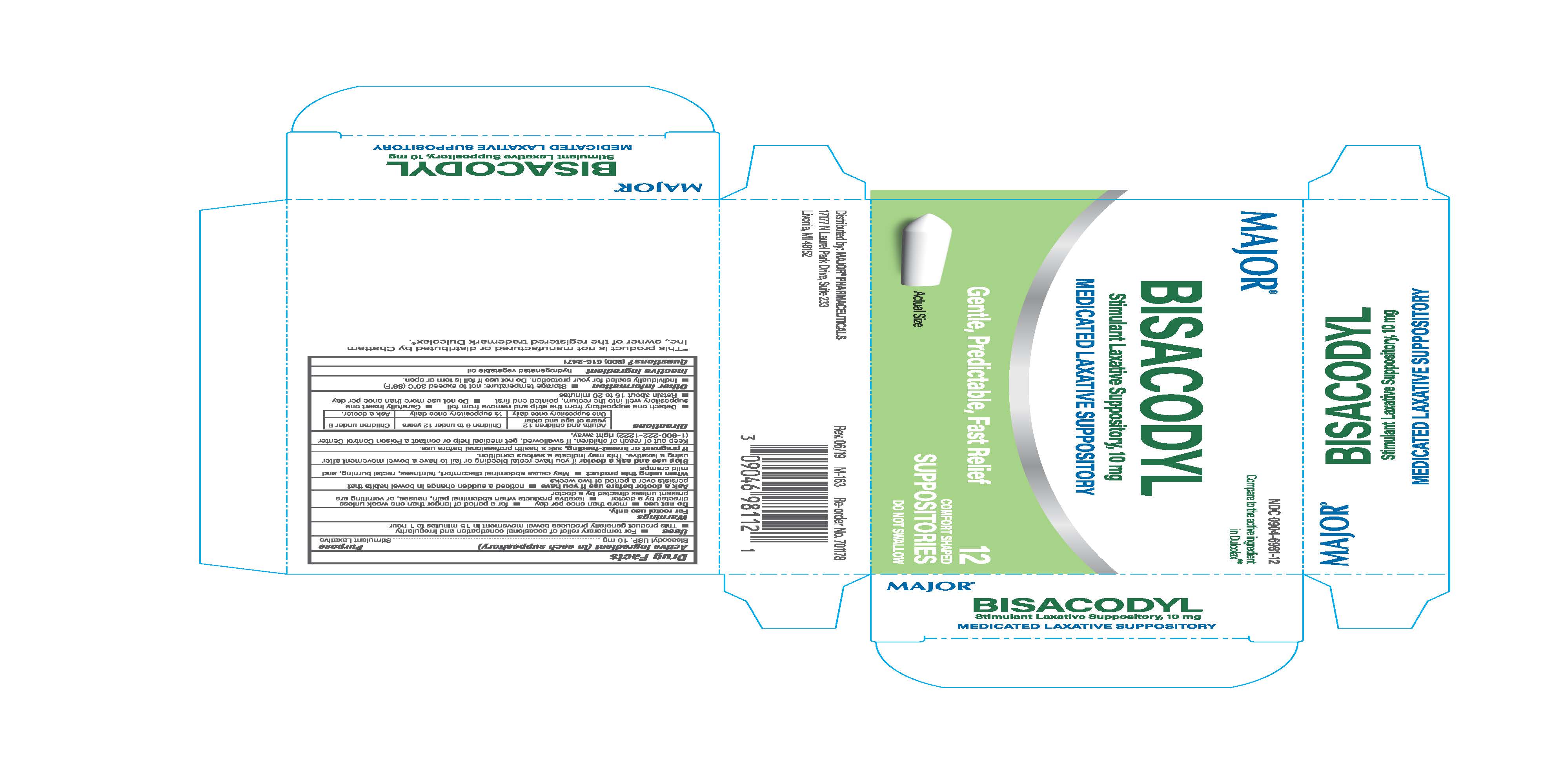
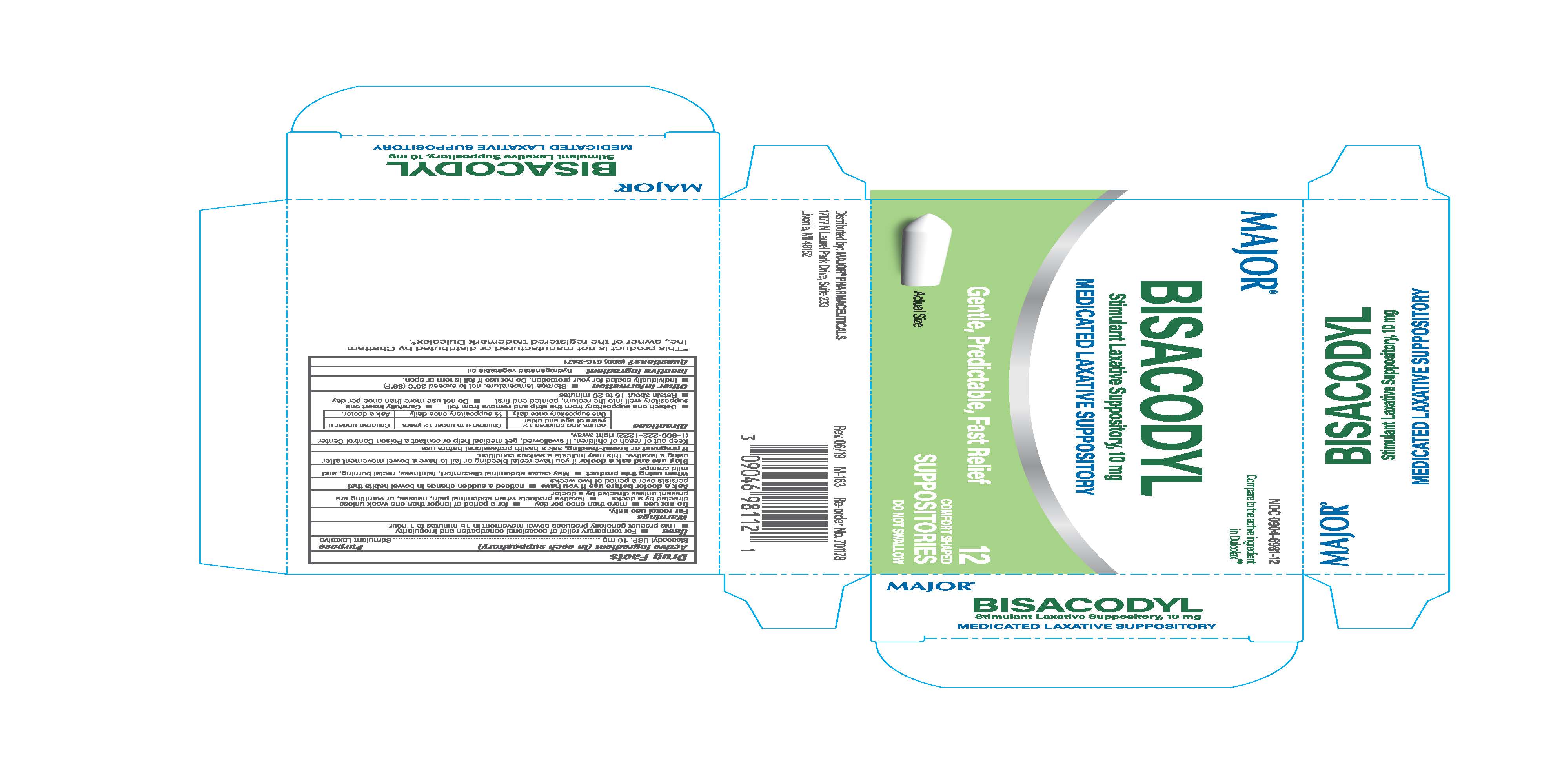
Label: LEADER GENTLE LAXATIVE- bisacodyl suppository suppository
- NDC Code(s): 0904-6981-12, 0904-6981-13
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each Suppository)
- Uses
- Warnings
- Do not use
- Ask a doctor
- When using this product
- Stop use and ask a doctor
- If pregnant or breast-feeding
- Keep out of reach of children
-
Directions
Adults and children 12 years of age and older Children 6 to under 12 years Children under 6
One suppository once daily 1/2 suppository once daily Ask doctor.
-Detach one suppository from the strip and remove from foil - Carefully insert one suppositry well into the rectum, pointed end first
- Retain about 15 to 20 minutes
- Other information
- Inactive ingredient
- Purpose
- Qutestions?
- Product Label
-
INGREDIENTS AND APPEARANCE
LEADER GENTLE LAXATIVE
bisacodyl suppository suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6981 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 10 mg in 2000 mg Inactive Ingredients Ingredient Name Strength PALM KERNEL OIL (UNII: B0S90M0233) Product Characteristics Color white Score Shape BULLET Size 32mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6981-12 1 in 1 CARTON 10/01/2019 1 NDC:0904-6981-13 120 mg in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 10/01/2019 01/01/2025 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(0904-6981)