Label: TICAGRELOR tablet
- NDC Code(s): 42794-039-02, 42794-039-06, 42794-039-10, 42794-039-22, view more
- Packager: Sigmapharm Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TICAGRELOR TABLETS safely and effectively. See full prescribing information for TICAGRELOR TABLETS. TICAGRELOR tablets, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: (A) BLEEDING RISK, (B) ASPIRIN DOSE AND TICAGRELOR TABLETS EFFECTIVENESS
A. BLEEDING RISK
- Ticagrelor tablets, like other antiplatelet agents, can cause significant, sometimes fatal bleeding ( 5.1, 6.1).
- Do not use ticagrelor tablets in patients with active pathological bleeding or a history of intracranial hemorrhage ( 4.1, 4.2).
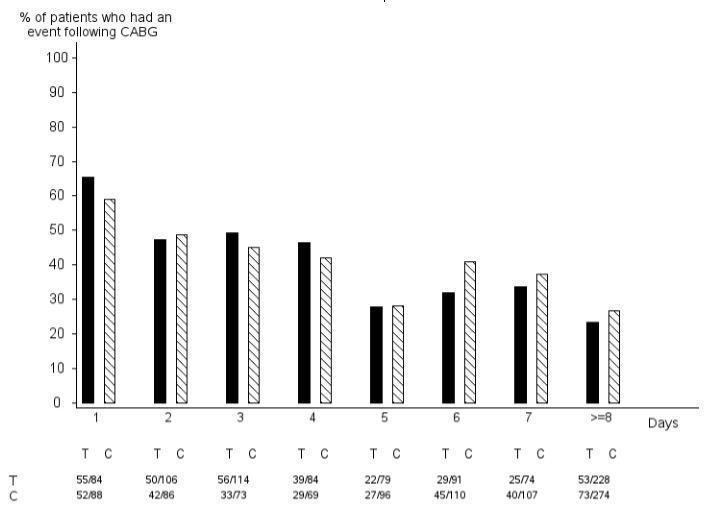
- Do not start ticagrelor tablets in patients undergoing urgent coronary artery bypass graft surgery (CABG) ( 5.1, 6.1).
- If possible, manage bleeding without discontinuing ticagrelor tablets. Stopping ticagrelor tablets increases the risk of subsequent cardiovascular events ( 5.4).
B. ASPIRIN DOSE AND TICAGRELOR TABLETS EFFECTIVENESS
- Maintenance doses of aspirin above 100 mg reduce the effectiveness of ticagrelor tablets and should be avoided ( 2.1, 5.2, 14.1).
-
1 INDICATIONS AND USAGE
Ticagrelor tablets are indicated to reduce the rate of cardiovascular death, myocardial infarction, and stroke in patients with acute coronary syndrome (ACS) or a history of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing - In the management of ACS, initiate ticagrelor tablets treatment with a 180 mg loading dose. Administer 90 mg twice daily during the ...
-
3 DOSAGE FORMS AND STRENGTHSTicagrelor tablets, 90 mg are supplied as yellow, round, biconvex, film coated tablets, debossed with “M” on one side and “ 90” on other side. Ticagrelor tablets, 60 mg are ...
-
4 CONTRAINDICATIONS4.1 History of Intracranial Hemorrhage - Ticagrelor tablets are contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this ...
-
5 WARNINGS AND PRECAUTIONS5.1 General Risk of Bleeding - Drugs that inhibit platelet function including ticagrelor tablets increase the risk of bleeding - [see Adverse Reactions ...
-
6 ADVERSE REACTIONSThe following adverse reactions are also discussed elsewhere in the labeling: Bleeding - [see Warnings and Precautions ( 5.1)] Dyspnea ...
-
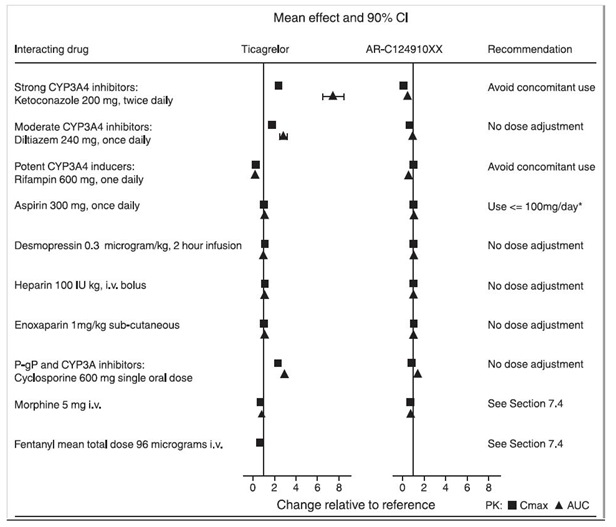
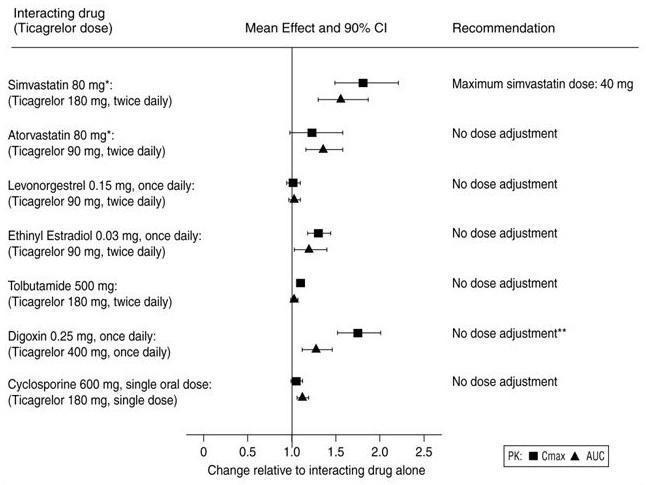
7 DRUG INTERACTIONS7.1 Strong CYP3A Inhibitors - Strong CYP3A inhibitors substantially increase ticagrelor exposure and so increase the risk of dyspnea, bleeding, and other adverse events. Avoid use of strong ...
-
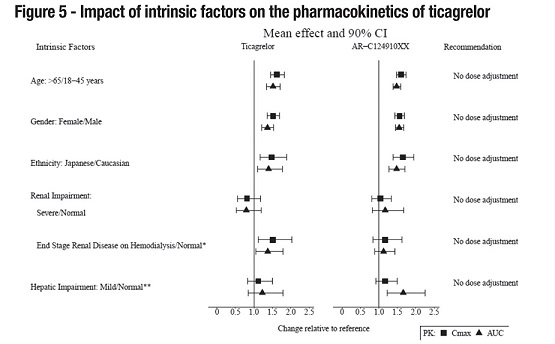
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from case reports with ticagrelor tablets use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or ...
-
10 OVERDOSAGEThere is currently no known treatment to reverse the effects of ticagrelor, and ticagrelor is not dialyzable. Treatment of overdose should follow local standard medical practice. Bleeding is the ...
-
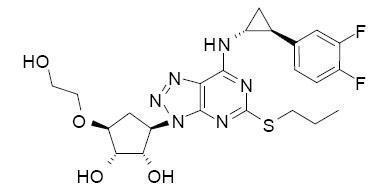
11 DESCRIPTIONTicagrelor tablets contain ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y - 12 ADP-receptor. Chemically it is (1 ...
-
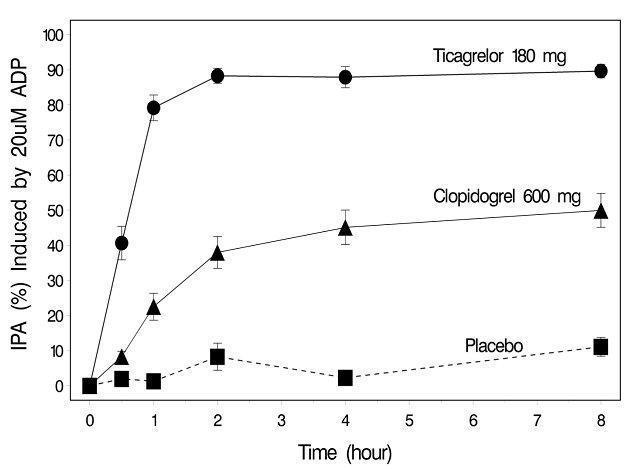
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ticagrelor and its major metabolite reversibly interact with the platelet P2Y - 12 ADP-receptor to prevent signal transduction and platelet activation ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Ticagrelor was not carcinogenic in the mouse at doses up to 250 mg/kg/day or in the male rat at doses up to 120 ...
-
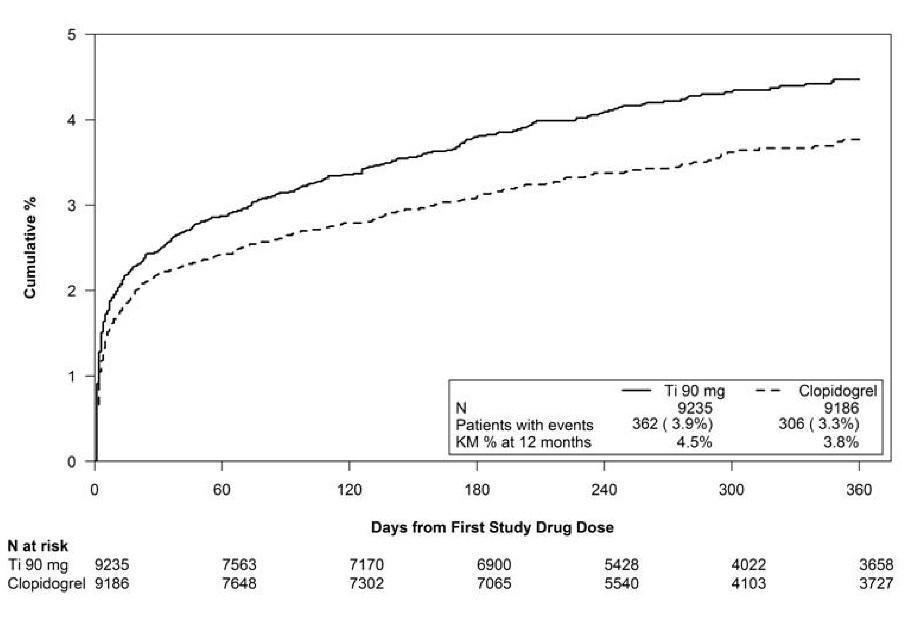
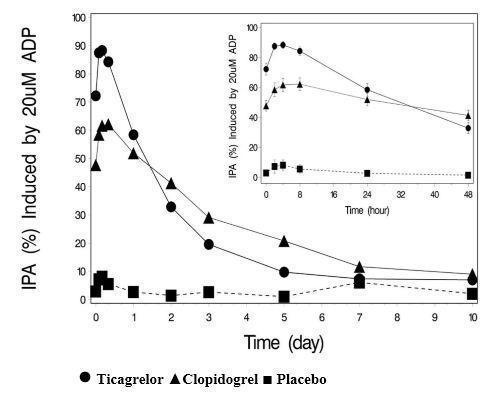
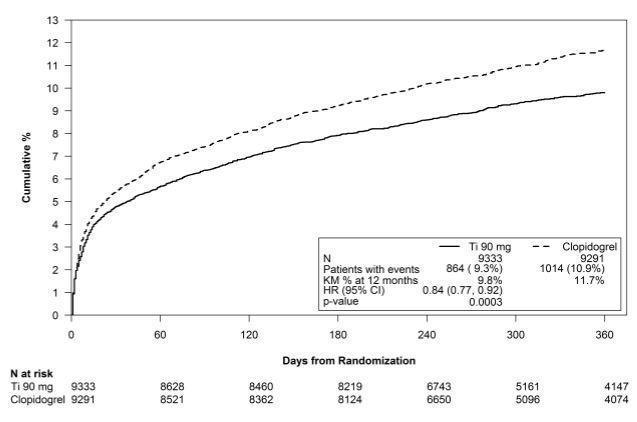
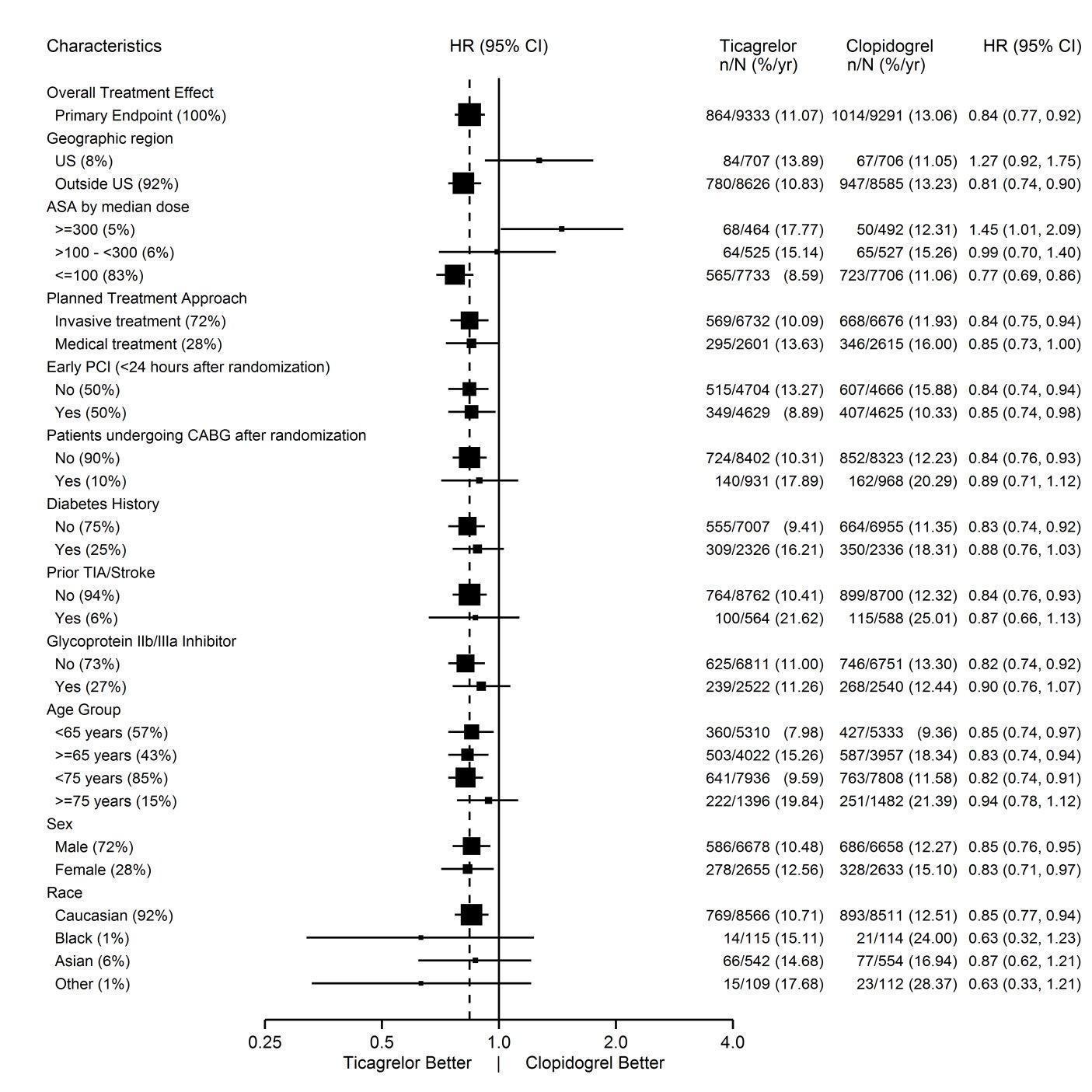
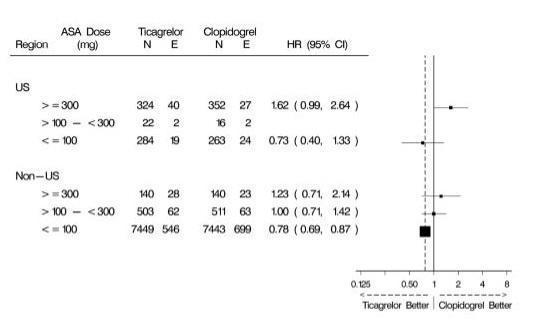
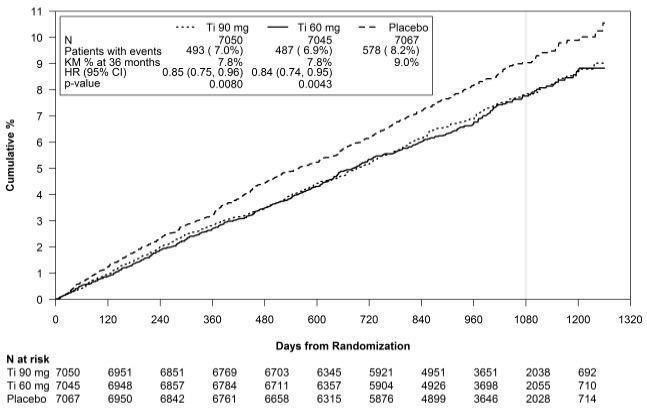
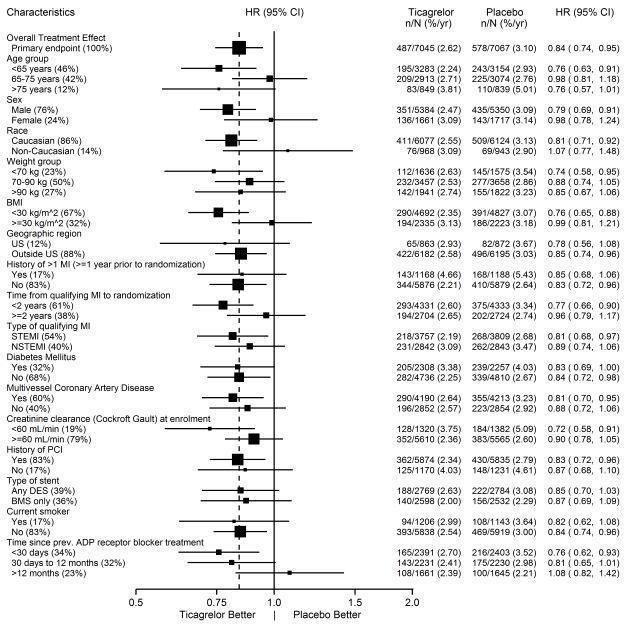
14 CLINICAL STUDIES14.1 Acute Coronary Syndromes and Secondary Prevention after Myocardial Infarction - PLATO - PLATO was a randomized double-blind study comparing ticagrelor (N=9333) to clopidogrel (N=9291), both ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTicagrelor Tablets, 90 mg are supplied as yellow, round, biconvex, film coated tablets, debossed with “M” on one side and “ 90” on other side: Bottles of 60 – NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Advise patients daily doses of aspirin should not exceed 100 mg and to avoid taking any other medications that ...
-
MEDICATION GUIDETicagrelor (TYE-ka-GREL-or) Tablets - What is the most important information I should know about ticagrelor tablets? Ticagrelor tablets are used to lower your chance of having a heart attack ...
-
TICAGRELOR TABLETS, 90 MG CONTAINER LABEL - 60 TABLETSNDC 42794- 039-10 - 60 Tablets - Ticagrelor Tablets - 90 mg - Rx only - Dispense the accompanying Medication Guide to each patient. Sigmapharm Laboratories, LLC
-
TICAGRELOR TABLETS, 90 MG CONTAINER LABEL - 180 TABLETSNDC 42794- 039-24 - 180 Tablets - Ticagrelor Tablets - 90 mg - Rx only - Dispense the accompanying Medication Guide to each patient. Sigmapharm Laboratories, LLC
-
TICAGRELOR TABLETS, 90 MG CONTAINER LABEL - 1000 TABLETSNDC 42794- 039-06 - 1000 Tablets - Ticagrelor Tablets - 90 mg - Rx only - Dispense the accompanying Medication Guide to each patient. Sigmapharm Laboratories, LLC
-
TICAGRELOR TABLETS, 60 MG CONTAINER LABEL - 60 TABLETSNDC 42794- 040-10 - 60 Tablets - Ticagrelor Tablets - 60 mg - Rx only - Dispense the accompanying Medication Guide to each patient. Sigmapharm Laboratories, LLC
-
TICAGRELOR TABLETS, 60 MG CONTAINER LABEL - 1000 TABLETSNDC 42794- 040-06 - 1000 Tablets - Ticagrelor Tablets - 60 mg - Rx only - Dispense the accompanying Medication Guide to each patient. Sigmapharm Laboratories, LLC
-
INGREDIENTS AND APPEARANCEProduct Information