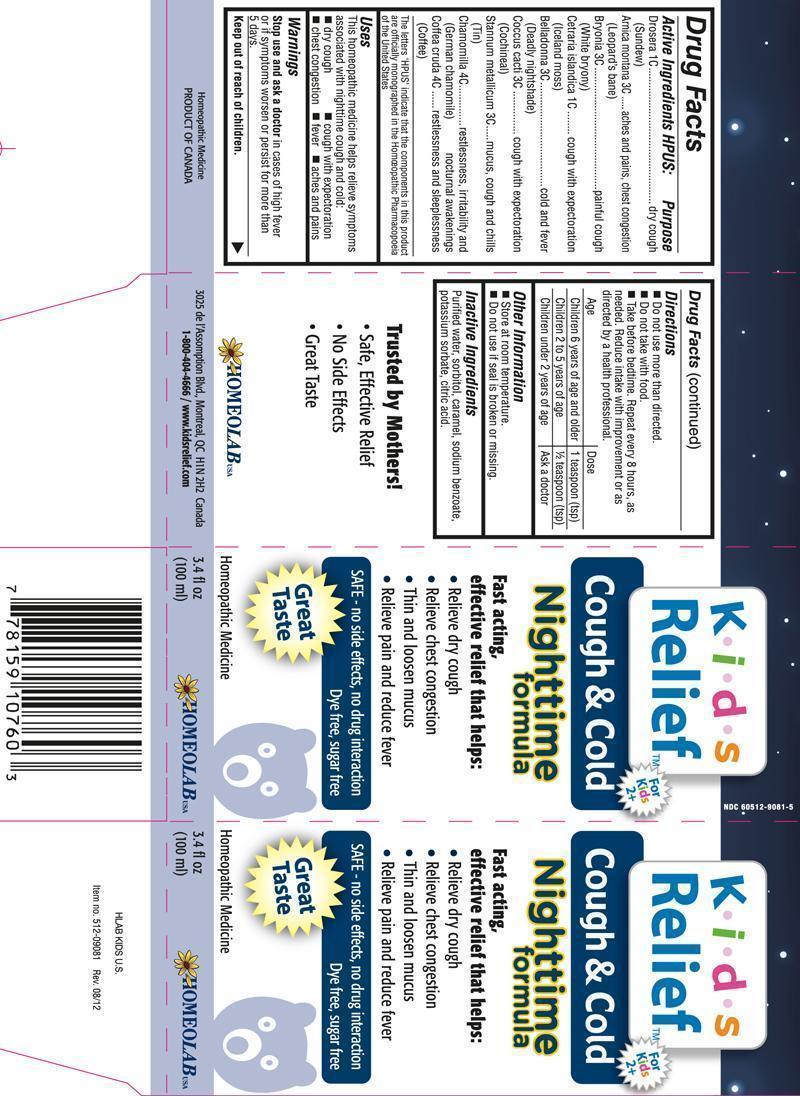
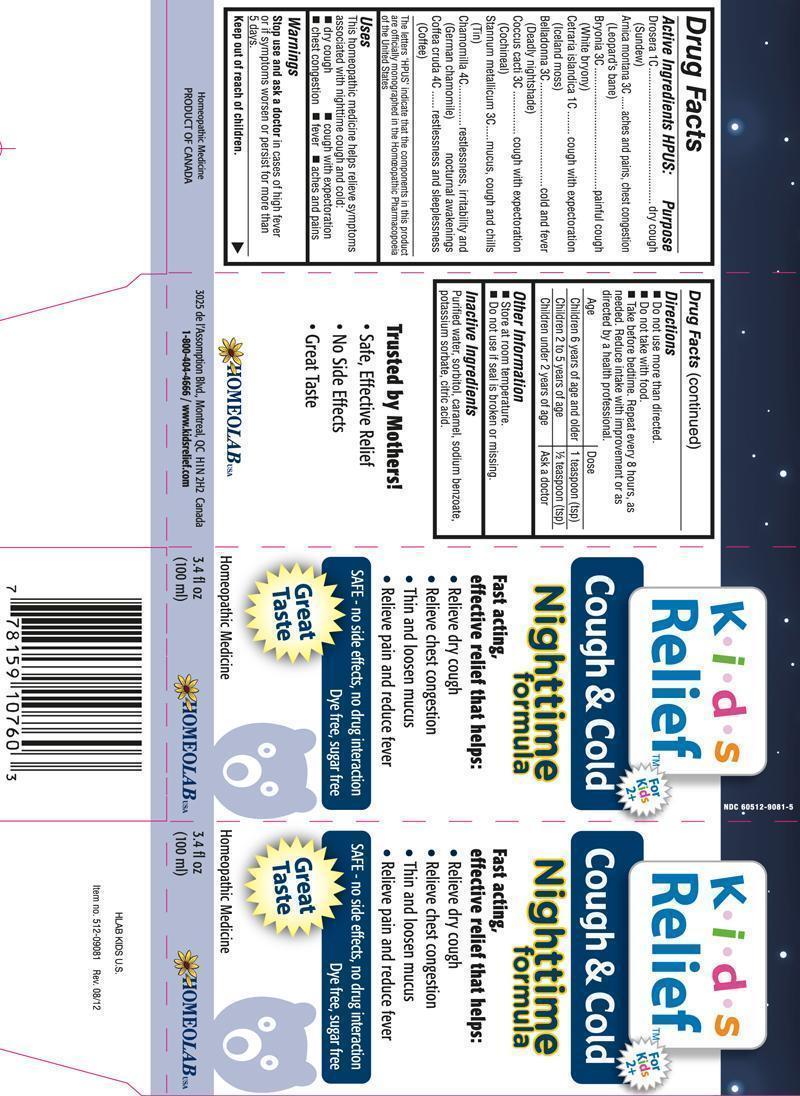
Label: COUGH AND COLD NIGHTTIME FORMULA KIDS RELIEF- drosera, arnica montana, bryonia, cetraria islandica, belladonna, coccus cacti, stannum metallicum, chamomilla, coffea cruda syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 60512-9021-4, 60512-9021-5, 60512-9021-9 - Packager: HOMEOLAB USA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 8, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- REFERENCES
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
Do not use more than directed.
Do not take with food.
Take before bedtime. Repeat every 8 hours, as needed. Reduce intake with improvement or as directed by a healthcare professional.
Age Dose Children 6 years of age and older 1 teaspoon Children 2 to 5 years of age 1/2 teaspoon Children under 2 years of age Ask a doctor - OTHER INFORMATION
- INACTIVE INGREDIENTS
- CARTON
-
INGREDIENTS AND APPEARANCE
COUGH AND COLD NIGHTTIME FORMULA KIDS RELIEF
drosera, arnica montana, bryonia, cetraria islandica, belladonna, coccus cacti, stannum metallicum, chamomilla, coffea cruda syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60512-9021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DROSERA ROTUNDIFOLIA (UNII: QR44N9XPJQ) (DROSERA ROTUNDIFOLIA - UNII:QR44N9XPJQ) DROSERA ROTUNDIFOLIA 1 [hp_C] in 100 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_C] in 100 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 3 [hp_C] in 100 mL CETRARIA ISLANDICA SUBSP. ISLANDICA (UNII: BJ7YPN79A1) (CETRARIA ISLANDICA SUBSP. ISLANDICA - UNII:BJ7YPN79A1) CETRARIA ISLANDICA SUBSP. ISLANDICA 1 [hp_C] in 100 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 3 [hp_C] in 100 mL PROTORTONIA CACTI (UNII: LZB7TFX1LT) (PROTORTONIA CACTI - UNII:LZB7TFX1LT) PROTORTONIA CACTI 3 [hp_C] in 100 mL TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 3 [hp_C] in 100 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 4 [hp_C] in 100 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 4 [hp_C] in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) CARAMEL (UNII: T9D99G2B1R) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60512-9021-9 250 mL in 1 BOTTLE 2 NDC:60512-9021-5 100 mL in 1 BOTTLE 3 NDC:60512-9021-4 30 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/28/2011 Labeler - HOMEOLAB USA INC (202032533) Establishment Name Address ID/FEI Business Operations HOMEOLAB USA INC 202032533 manufacture(60512-9021)