Label: NALOXONE HYDROCHLORIDE spray
- NDC Code(s): 71205-707-02
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 45802-811
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NALOXONE HYDROCHLORIDE NASAL SPRAY safely and effectively. See full prescribing information for NALOXONE HYDROCHLORIDE NASAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Naloxone HCl Nasal Spray is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. Naloxone HCl Nasal ...
-
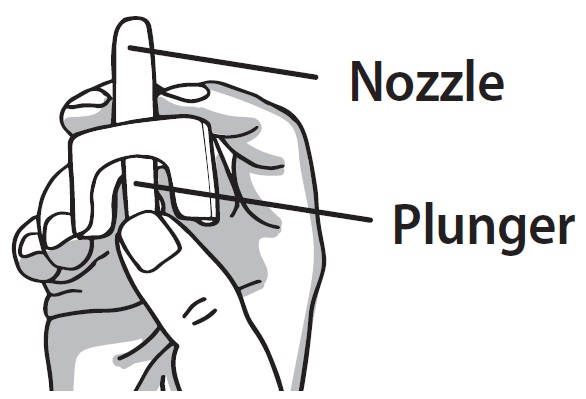
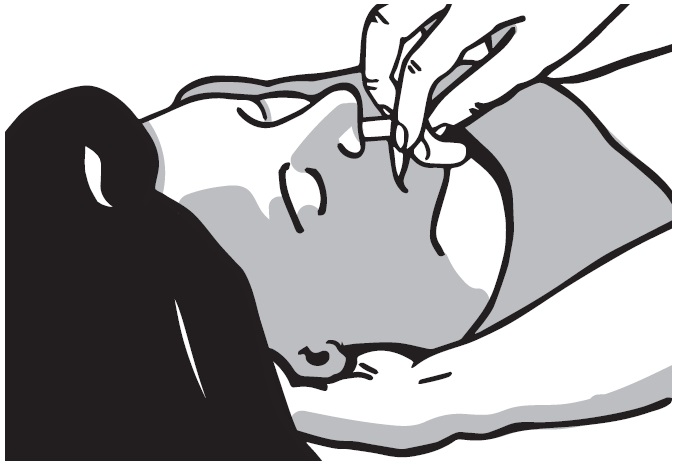
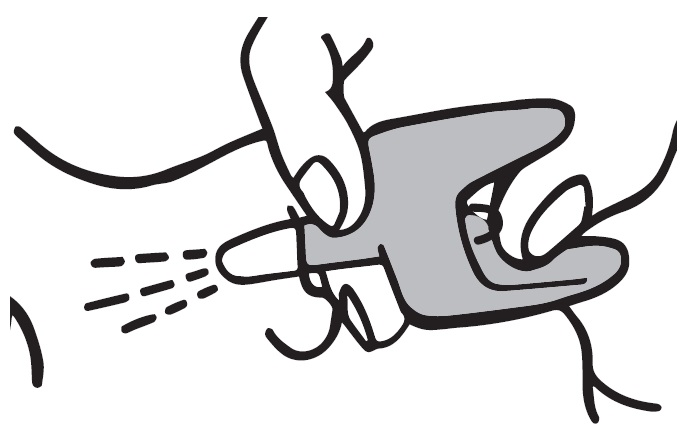
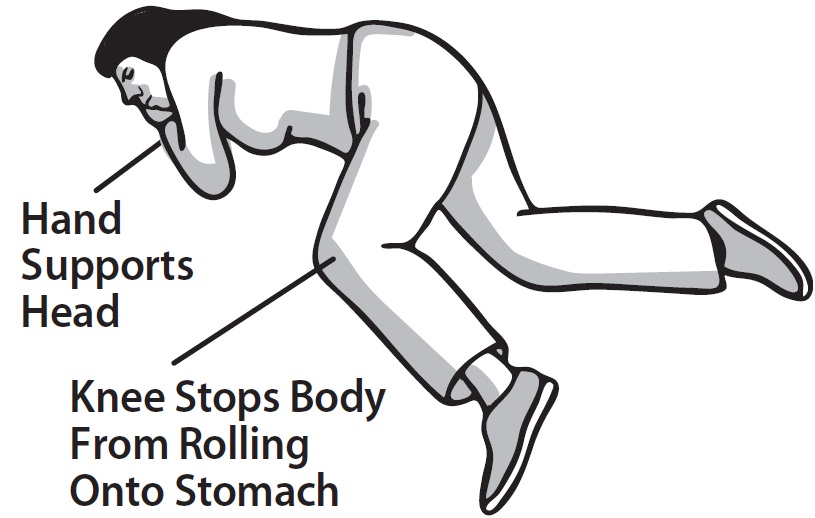
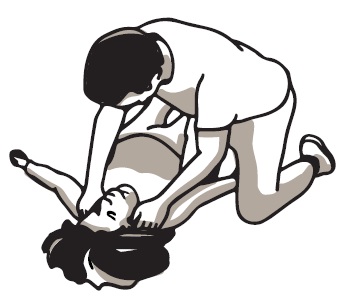
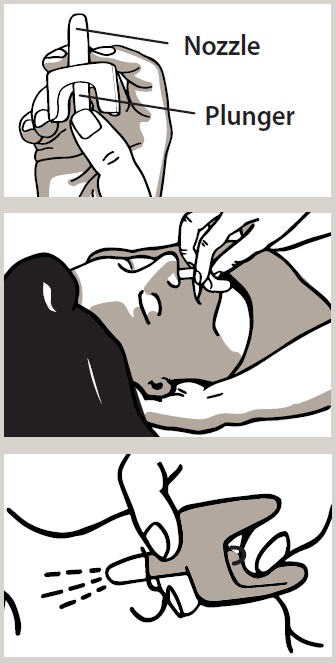
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Instructions - Naloxone HCl Nasal Spray is for intranasal use only. No additional device assembly is required. Because treatment of suspected opioid overdose must be ...
-
3 DOSAGE FORMS AND STRENGTHS Naloxone HCl Nasal Spray is supplied as a single-dose intranasal spray containing 4 mg of naloxone hydrochloride in 0.1 mL.
-
4 CONTRAINDICATIONS Naloxone HCl Nasal Spray is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or to any of the other ingredients.
-
5 WARNINGS AND PRECAUTIONS 5.1 Risk of Recurrent Respiratory and Central Nervous System Depression - The duration of action of most opioids may exceed that of Naloxone HCl Nasal Spray resulting in a return of respiratory ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed elsewhere in the labeling: • Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.3)] Because clinical studies are ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - The limited available data on naloxone use in pregnant women are not sufficient to inform a drug- associated risk. However, there are clinical considerations [see ...
-
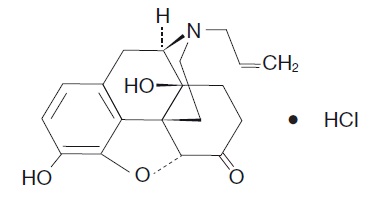
11 DESCRIPTION Naloxone HCl Nasal Spray is a pre-filled, single dose intranasal spray. Chemically, naloxone hydrochloride is the hydrochloride salt of 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one ...
-
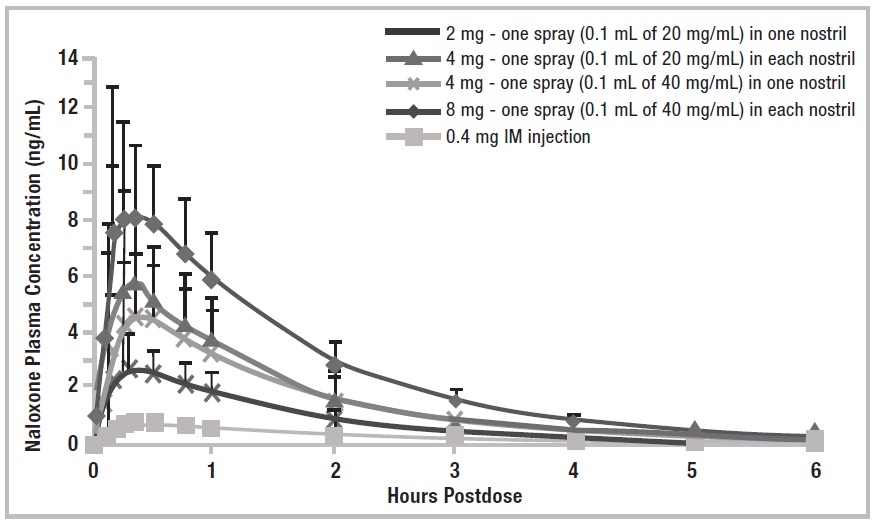
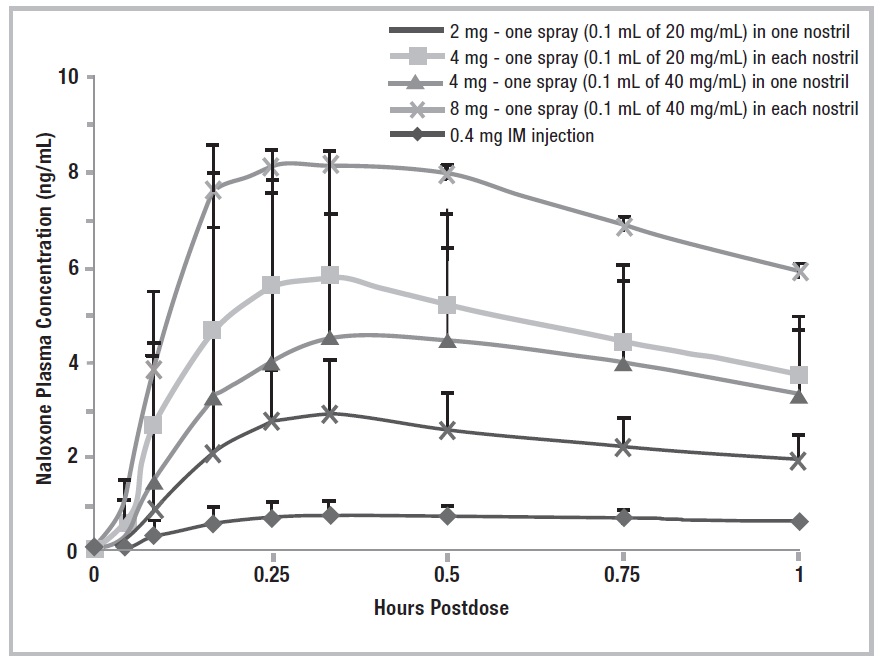
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites. Naloxone hydrochloride reverses the effects of ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - Naloxone HCl Nasal Spray 4 mg is supplied as Carton containing two (2) blister packages - (NDC 71205-707-02) each with a single spray device. Naloxone HCl Nasal Spray is not ...
-
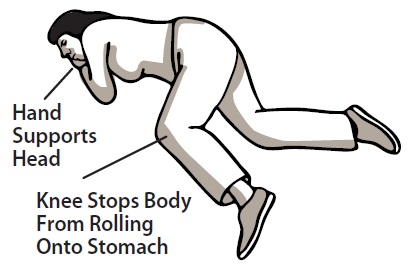
17 PATIENT COUNSELING INFORMATION Advise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Recognition of Opioid Overdose - Inform patients and ...
-
PATIENT INFORMATION PATIENT INFORMATION - Naloxone (nal-OKS-one) Hydrochloride Nasal Spray - You and your family members or caregivers should read this Patient Information leaflet before an opioid emergency ...
-
Instructions for Use Instructions for Use - Naloxone (nal-OKS-one) Hydrochloride Nasal Spray - You and your family members or caregivers should read the Instructions for Use that comes with Naloxone HCl Nasal Spray ...
-
QUICK START GUIDE Naloxone HCl Nasal Spray 4 mg - QUICK START GUIDE - Opioid Overdose Response Instructions - Use Naloxone Hydrochloride Nasal Spray for known or suspected opioid overdose in adults and ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Rx Only - NDC 71205-707-02 - Naloxone HCl Nasal Spray - 4 mg - Important: FOR USE IN THE NOSE ONLY. Use Naloxone HCl Nasal Spray for known or suspected opioid overdose in adults and children. Do not ...
-
INGREDIENTS AND APPEARANCEProduct Information