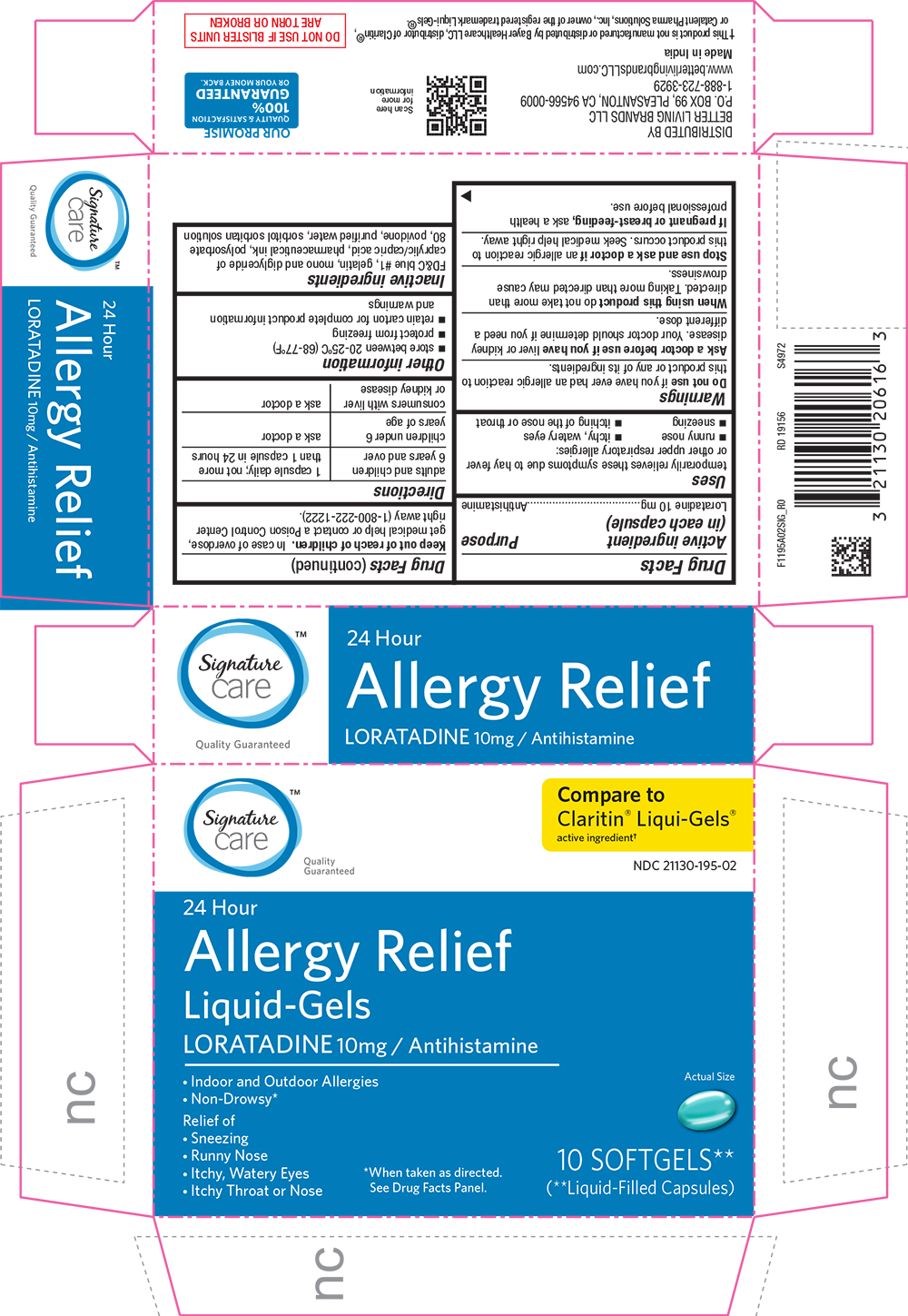
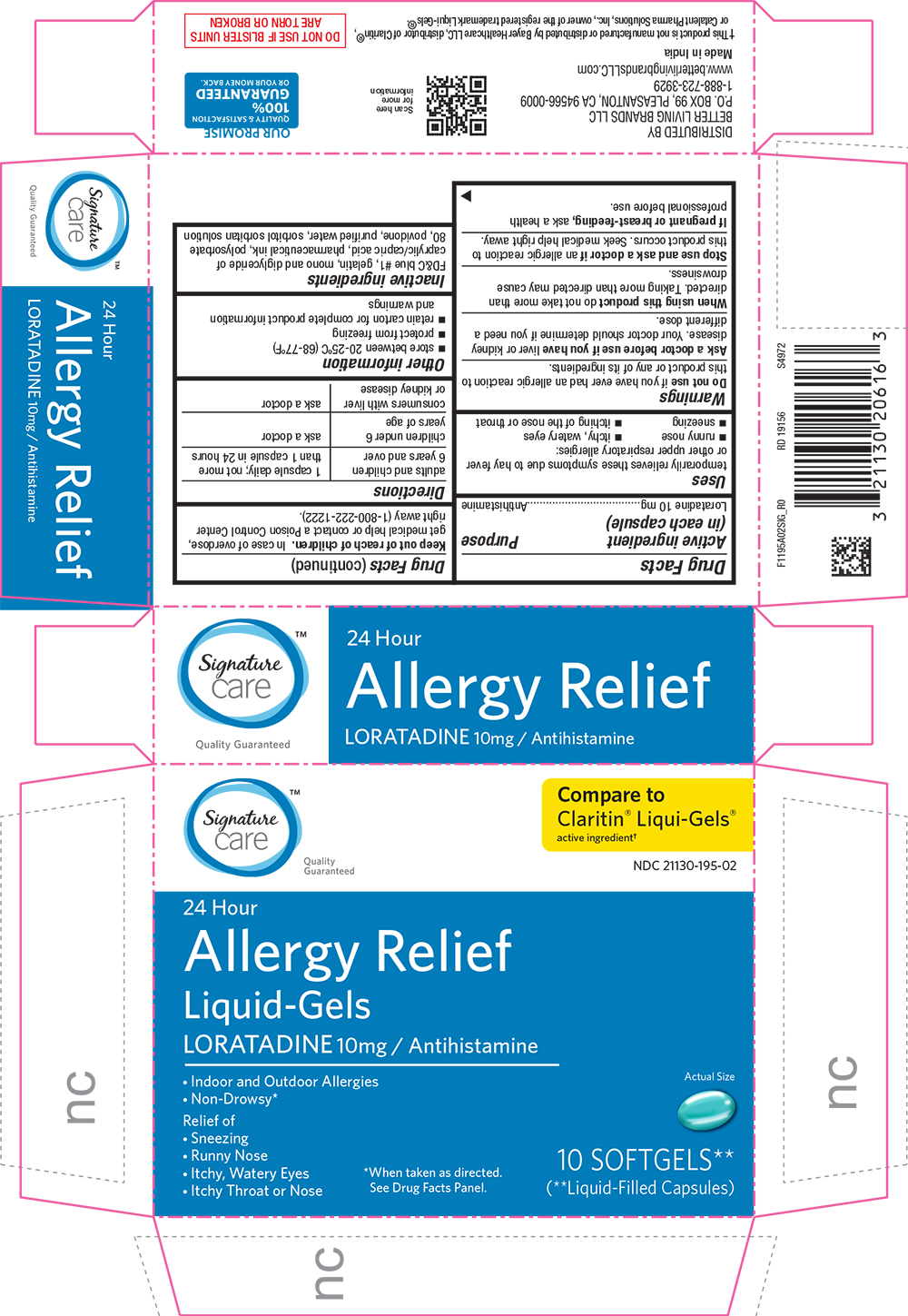
Label: ALLERGY RELIEF- loratadine capsule, liquid filled
- NDC Code(s): 21130-195-02
- Packager: Safeway
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 8, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
Signature Care
Quality Guaranteed
Compare to Claritin(R) Liqui-Gels(R) active ingredient
NDC 21130-195-02
24 Hour
Allergy Relief
Liquid-Gels
LORATADINE 10mg / Antihistamine
Indoor and Outdoor Allergies
Non-Drowsy*
Actual Size
Relief of
Sneezing, Runny Nose, Itchy, Watery Eyes, Itchy Throat or Nose
*When taken as directed. See Drug Facts Panel.
10 SOFTGELS**
(**Liquid-Filled Capsules)
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
loratadine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-195 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) CAPRIC ACID (UNII: 4G9EDB6V73) POVIDONE K30 (UNII: U725QWY32X) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SORBITAN (UNII: 6O92ICV9RU) Product Characteristics Color blue (Light Blue) Score no score Shape OVAL (oval shaped) Size 3mm Flavor Imprint Code 21 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-195-02 1 in 1 CARTON 10/01/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206214 10/01/2019 Labeler - Safeway (009137209)