Label: ZYNTEGLO- betibeglogene autotemcel suspension
- NDC Code(s): 73554-3111-1
- Packager: bluebird bio, Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZYNTEGLO® safely and effectively. See full prescribing information for ZYNTEGLO. ZYNTEGLO (betibeglogene autotemcel) suspension ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZYNTEGLO is indicated for the treatment of adult and pediatric patients with β-thalassemia who require regular red blood cell (RBC) transfusions.
-
2 DOSAGE AND ADMINISTRATIONFor autologous use only. For one-time single-dose intravenous use only. 2.1 Dose - ZYNTEGLO is provided as a single dose for infusion containing a suspension of CD34+ cells in one or more ...
-
3 DOSAGE FORMS AND STRENGTHSZYNTEGLO is a cell suspension for intravenous infusion. ZYNTEGLO is composed of up to four infusion bags which contain 2.0 to 20 × 106 cells/mL suspended in cryopreservation solution [see How ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Delayed Platelet Engraftment - Delayed platelet engraftment has been observed with ZYNTEGLO treatment. Bleeding risk is increased prior to platelet engraftment and may continue after ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in the labeling: Delayed Platelet Engraftment [see Warnings and Precautions (5.1)] Risk of Neutrophil Engraftment Failure [see Warnings ...
-
7 DRUG INTERACTIONSNo formal drug interaction studies have been performed. ZYNTEGLO is not expected to interact with the hepatic cytochrome P-450 family of enzymes or drug transporters. 7.1 Live Vaccines - Follow ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with ZYNTEGLO administration in pregnant women. Consider the risks associated with myeloablative conditioning agents on pregnancy and ...
-
11 DESCRIPTIONZYNTEGLO (betibeglogene autotemcel) is a βA-T87Q-globin gene therapy consisting of autologous CD34+ cells, containing hematopoietic stem cells (HSCs), transduced with BB305 LVV encoding ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ZYNTEGLO adds functional copies of a modified β-globin gene into patients' hematopoietic stem cells (HSCs) through transduction of autologous CD34+ cells with BB305 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity studies have been performed with ZYNTEGLO. Intravenous administration of ZYNTEGLO in a mouse model of β-thalassemia ...
-
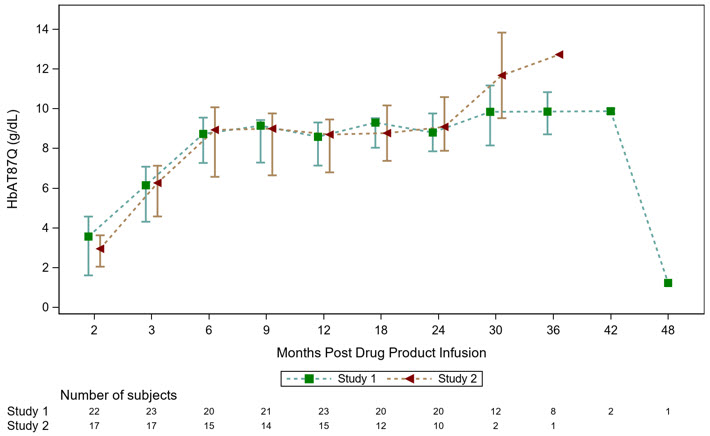
14 CLINICAL STUDIESThe efficacy of ZYNTEGLO was evaluated in 2 ongoing Phase 3 open-label, single-arm, 24-month, multicenter studies (Study 1 and Study 2) in 41 patients aged 4 to 34 years with β-thalassemia ...
-
15 REFERENCES1 Lai, X., Liu, L., Zhang, Z. et al. Hepatic veno-occlusive disease/sinusoidal obstruction syndrome after hematopoietic stem cell transplantation for thalassemia major: incidence, management, and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZYNTEGLO is supplied in up to four infusion bags containing a frozen suspension of genetically modified autologous cells, enriched for CD34+ cells. Each bag contains approximately 20 mL. Each ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Ensure that patients understand the risk of manufacturing failure. In case of manufacturing failure or the need ...
-
SPL UNCLASSIFIED SECTIONManufactured for: bluebird bio, Inc. Somerville, MA 02145 - US License No 2160
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ZYNTEGLO® (pronounced zin-TEH-glo) (betibeglogene autotemcel) This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: August ...
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Patient Identifier Labelbetibeglogene autotemcel - zynteglo™ Suspension for IV infusion - 20 mL containing 2.0 to 20 x 106 cells/mL - (1.7 to 20 x 106 CD34+ cells/mL) Confirm Patient Identifiers - Last Name: $LastName$ First ...
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Labelbetibeglogene autotemcel - zynteglo™ Suspension for IV infusion - 20 mL containing 2.0 to 20 x 106 cells/mL - (1.7 to 20 x 106 CD34+ cells/mL) For autologous use only. For intravenous use only. Rx ...
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Cassette Labelbetibeglogene autotemcel - zynteglo™ Suspension for IV infusion - 20 mL containing 2.0 to 20 x 106 cells/mL - (1.7 to 20 x 106 CD34+ cells/mL) For autologous use only. For intravenous use only. Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information