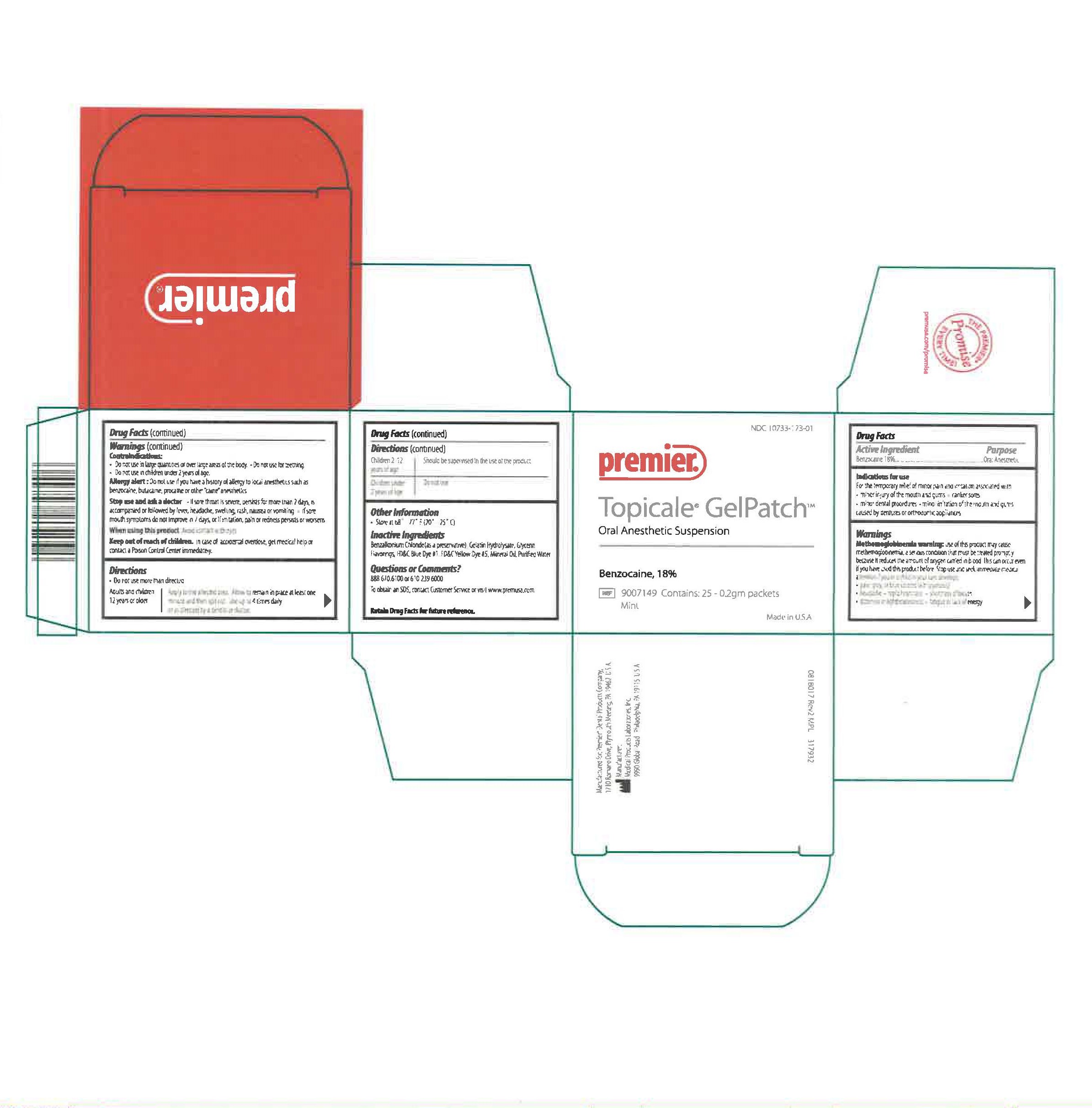
Label: TOPICALE- benzocaine patch
- NDC Code(s): 10733-173-01
- Packager: Medical Products Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientBenzocaine 18%
-
PURPOSEOral Anesthetic
-
INDICATIONS & USAGEFor the temporary relief of minor pain and irritation associated with minor injury of the mouth and gums, canker sores, minor dental procedures, minor irritation of the mouth and gums caused by ...
-
WARNINGSMethemoglobinemia warning: Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can ...
-
DOSAGE & ADMINISTRATIONDo not use more than directed. Adults and children 12 years or older - Apply to the affected area. Allow to remain in place at least one minute and the spit out. Use up to 4 times daily or as ...
-
OTHER SAFETY INFORMATIONStore at 68º to 77º F (20º - 25º C)
-
INACTIVE INGREDIENTBenzalkonium Chloride (as a preservative), Gelatin Hydrolysate, Glycerin, Flavorings,FD&C Blue Dye # 1, FD&C Yellow Dye # 5, Mineral Oil, Purified Water
-
INFORMATION FOR OWNERS/CAREGIVERSNDC 10733-173-01 - Premier - Topicale GelPatch - Oral Anesthetic Suspension - Benzocaine, 18 % REF 9007149 Contains: 25 - 0.2gm Packets - Mint - Made in U.S.A. Manufactured for: Premier® Dental ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information