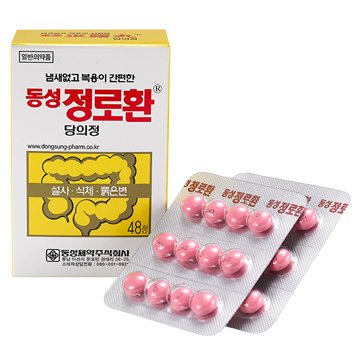
Label: CHEONG RO HWAN SUGAR COATED- creosote tablet, sugar coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0030-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Ask a doctor before use if you have taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
■ Do not take more than 4 pills in 24 hours
■ Do not use the maximum dosage for more than 1 weeks
Keep out of the reach of children. This package contains enough drug to seriously harm a child
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHEONG RO HWAN SUGAR COATED
creosote tablet, sugar coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 22.5 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor Imprint Code CHT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0030-1 48 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/20/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/13/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 manufacture(72689-0030) , label(72689-0030)