Label: GLYCOPYRROLATE ORAL SOLUTION liquid
- NDC Code(s): 68022-0156-1
- Packager: Suven Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
GLYCOPYRROLATE ORAL SOLUTIONHIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use GLYCOPYRROLATE ORAL SOLUTION safely and effectively. See full prescribing information for ...
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GLYCOPYRROLATE ORAL SOLUTION safely and effectively. See full prescribing information for GLYCOPYRROLATE ORAL SOLUTION.
GLYCOPYRROLATE ORAL SOLUTION
Initial U.S. Approval: 1961
--------------------------INDICATIONS AND USAGE----------------------
GLYCOPYRROLATE ORAL SOLUTION 1MG/5ML is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling (e.g., cerebral palsy). (1)
---------------------DOSAGE AND ADMINISTRATION-----------------
Initiate dosing at 0.02 mg/kg three times daily and titrate in increments of 0.02 mg/kg every 5-7 days, based on therapeutic response and adverse reactions. (2)
Maximum recommended dose is 0.1 mg/kg three times daily, not to exceed 1.5-3 mg per dose based upon weight. (2)
Administer at least one hour before or two hours after meals. (2)-------------------DOSAGE FORMS AND STRENGTHS-----------------
1 mg/5 mL, oral solution in 16 ounce bottles. (3)
-------------------------CONTRAINDICATIONS ---------------------------
Medical conditions that preclude anticholinergic therapy. (4)
Concomitant use of solid oral dosage forms of potassium chloride. (4)------------------WARNINGS AND PRECAUTIONS ---------------------
Constipation or intestinal pseudo-obstruction: May present as abdominal distention, pain, nausea, or vomiting. Assess patients for constipation, particularly within 4-5 days of initial dosing or after a dose increase. (5.1)
Incomplete mechanical intestinal obstruction: May present as diarrhea. If obstruction is suspected, discontinue GLYCOPYRROLATE ORAL SOLUTION 1MG/5ML and evaluate. (5.2)
High ambient temperature: To reduce the risk of heat prostration, avoid high temperatures. (5.3)--------------------------ADVERSE REACTIONS ---------------------------
The most common adverse reactions (incidence ≥30%) are dry mouth, vomiting, constipation, flushing, and nasal congestion. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Suven Life Sciences Limited at (732) 419-3654 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-------------------------DRUG INTERACTIONS-----------------------------
Digoxin tablets: Use with glycopyrrolate can increase digoxin serum levels. Monitor patients and consider use of alternative dosage forms of digoxin. (7)
Amantadine: Effects of glycopyrrolate may be increased with concomitant administration of amantadine. Consider decreasing the dose of glycopyrrolate during concomitant use. (7)
Atenolol or metformin: Glycopyrrolate may increase serum levels of atenolol or metformin. Consider dose reduction when used with glycopyrrolate. (7)
Haloperidol or levodopa: Glycopyrrolate may decrease serum levels of haloperidol or levodopa. Consider a dose increase when used with glycopyrrolate. (7)------------------USE IN SPECIFIC POPULATIONS --------------------
Pediatric use: The safety and effectiveness of glycopyrrolate has not been established in patients less than 3 years of age. (8.4)
Renal impairment: Use GLYCOPYRROLATE ORAL SOLUTION 1MG/5ML with caution in patients with renal impairment. (8.6)See 17 for PATIENT COUNSELING INFORMATION and FDA-
approved patient labeling
Revised: 07/2021
1 INDICATIONS AND USAGE
Glycopyrrolate oral solution is indicated to reduce chronic severe drooling in patients aged 3 to 16 years with neurologic conditions associated with problem drooling (e.g., cerebral palsy)
11 DESCRIPTION
Glycopyrrolate oral solution is an anticholinergic drug available as an oral solution containing 1 mg glycopyrrolate per 5 mL. The chemical name for glycopyrrolate is pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl) oxy]-1,1-dimethyl-bromide. The chemical structure is:
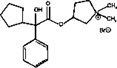
The empirical formula for glycopyrrolate oral solution is C 19H 28BrNO 3 and the molecular weight is 398.33. The inactive ingredients in glycopyrrolate oral solution are: citric acid, glycerin, sour cherry flavor, ethyl paraben, propylene glycol, propyl paraben, saccharin sodium, sodium citrate, sorbitol solution, and purified water
2 DOSAGE AND ADMINISTRATION
Glycopyrrolate oral solution must be measured and administered with an accurate measuring device [see Patient Counseling Information (17)].
Initiate dosing at 0.02 mg/kg orally three times daily and titrate in increments of 0.02 mg/kg every 5-7 days based on therapeutic response and adverse reactions. The maximum recommended dosage is 0.1 mg/kg three times daily not to exceed 1.5-3 mg per dose based upon weight. For greater detail, see Table 1.
During the four-week titration period, dosing can be increased with the recommended dose titration schedule while ensuring that the anticholinergic adverse events are tolerable. Prior to each increase in dose, review the tolerability of the current dose level with the patient’s caregiver.
Glycopyrrolate oral solution should be dosed at least one hour before or two hours after meals.
The presence of high fat food reduces the oral bioavailability of glycopyrrolate oral solution if taken shortly after a meal [see Clinical Pharmacology (12.3)].
Table 1: Recommended Dose Titration Schedule (each dose to be given three times daily) Weight Dose Level 1 Dose Level 2 Dose Level 3 Dose Level 4 Dose Level 5 kg lbs (~0.02 mg/kg) (~0.04 mg/kg) (~0.06 mg/kg) (~0.08 mg/kg) (~0.1 mg/kg) 13-17 27-38 0.3 mg 1.5 mL 0.6 mg 3 mL 0.9 mg 4.5 mL 1.2 mg 6 mL 1.5 mg 7.5 mL 18-22 39-49 0.4 mg 2 mL 0.8 mg 4 mL 1.2 mg 6 mL 1.6 mg 8 mL 2.0 mg 10 mL 23-27 50-60 0.5 mg 2.5 mL 1.0 mg 5 mL 1.5 mg 7.5 mL 2.0 mg 10 mL 2.5 mg 12.5 mL 28-32 61-71 0.6 mg 3 mL 1.2 mg 6 mL 1.8 mg 9 mL 2.4 mg 12 mL 3.0 mg 15 mL 33-37 72-82 0.7 mg 3.5 mL 1.4 mg 7 mL 2.1 mg 10.5 mL 2.8 mg 14 mL 3.0 mg 15 mL 38-42 83-93 0.8 mg 4 mL 1.6 mg 8 mL 2.4 mg 12 mL 3.0 mg 15 mL 3.0 mg 15 mL 43-47 94-104 0.9 mg 4.5 mL 1.8 mg 9 mL 2.7 mg 13.5 mL 3.0 mg 15 mL 3.0 mg 15 mL ≥48 ≥105 1.0 mg 5 mL 2.0 mg 10 mL 3.0 mg 15 mL 3.0 mg 15 mL 3.0 mg 15 mL 3 DOSAGE FORMS AND STRENGTHS
Glycopyrrolate oral solution is available as a 1 mg/5 mL clear, cherry-flavored solution for oral administration in 16 ounce bottles.
4 CONTRAINDICATIONS
Glycopyrrolate oral solution is contraindicated in:
- Patients with medical conditions that preclude anticholinergic therapy (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis).
- Patients taking solid oral dosage forms of potassium chloride. The passage of potassium chloride tablets through the gastrointestinal (GI) tract may be arrested or delayed with coadministration of glycopyrrolate oral solution.
5 WARNINGS AND PRECAUTIONS
5.1 Constipation or Intestinal Pseudo-obstruction
Constipation is a common dose-limiting adverse reaction which sometimes leads to glycopyrrolate discontinuation [see Adverse Reactions (6.1)]. Assess patients for constipation, particularly within 4-5 days of initial dosing or after a dose increase. Intestinal pseudo-obstruction has been reported and may present as abdominal distention, pain, nausea or vomiting.
5.2 Incomplete Mechanical Intestinal Obstruction
Diarrhea may be an early symptom of incomplete mechanical intestinal obstruction, especially in patients with ileostomy or colostomy. If incomplete mechanical intestinal obstruction is suspected, discontinue treatment with glycopyrrolate oral solution and evaluate for intestinal obstruction.
5.3 High Ambient Temperatures
In the presence of high ambient temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with the use of anticholinergic drugs such as glycopyrrolate oral solution. Advise patients/caregivers to avoid exposure of the patient to hot or very warm environmental temperatures.
5.4 Operating Machinery or an Automobile
Glycopyrrolate oral solution may produce drowsiness or blurred vision. As appropriate for a given age, warn the patient not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery, or performing hazardous work while taking glycopyrrolate oral solution.
5.5 Anticholinergic Drug Effects
Use glycopyrrolate oral solution with caution in patients with conditions that are exacerbated by anticholinergic drug effects including:
- Autonomic neuropathy
- Renal disease
- Ulcerative colitis – Large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason may precipitate or aggravate “toxic megacolon”, a serious complication of the disease.
- Hyperthyroidism
- Coronary heart disease, congestive heart failure, cardiac tachyarrhythmias, tachycardia, and hypertension
- Hiatal hernia associated with reflux esophagitis, since anticholinergic drugs may aggravate this condition
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Constipation or intestinal pseudo-obstruction [see Warnings and Precautions (5.1)]
- Incomplete mechanical intestinal obstruction [see Warnings and Precautions (5.2)]
The most common adverse reactions reported with glycopyrrolate oral solution are dry mouth, vomiting, constipation, flushing, and nasal congestion
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to glycopyrrolate oral solution in 151 subjects, including 20 subjects who participated in an 8-week placebo-controlled study (Study 1) and 137 subjects who participated in a 24-week open-label study (six subjects who received glycopyrrolate oral solution in the placebo-controlled study and 131 new subjects).
Table 2 presents adverse reactions reported by ≥ 15% of glycopyrrolate oral solution -treated subjects from the placebo-controlled clinical trial.
Table 2: Adverse Reactions Occurring in ≥ 15% of Glycopyrrolate oral solution Treated Subjects and at a Greater Frequency than Placebo in Study 1 Glycopyrrolate oral solution (N=20)
n (%)
Placebo (N=18)
n (%)
Dry Mouth
8 (40%)
2 (11%)
Vomiting
8 (40%)
2 (11%)
Constipation
7 (35%)
4 (22%)
Flushing
6 (30%)
3 (17%)
Nasal Congestion
6 (30%)
2 (11%)
Headache
3 (15%)
1 (6%)
Sinusitis
3 (15%)
1 (6%)
Upper Respiratory Tract Infection
3 (15%)
0
Urinary Retention
3 (15%)
0
The following adverse reactions occurred at a rate of <2% of patients receiving glycopyrrolate oral solution in the open-label study.
Gastrointestinal: Abdominal distention, abdominal pain, stomach discomfort, chapped lips, flatulence, retching, dry tongue General Disorders: Irritability, pain
Infections: Pneumonia, sinusitis, tracheostomy infection, upper respiratory tract infection, urinary tract infection Investigations: Heart rate increase
Metabolism and Nutrition: Dehydration
Nervous System: Headache, convulsion, dysgeusia, nystagmus
Psychiatric: Agitation, restlessness, abnormal behavior, aggression, crying, impulse control disorder, moaning, mood altered Respiratory: Increased viscosity of bronchial secretion, nasal congestion, nasal dryness
Skin: Dry skin, pruritus, rash
Vascular: Pallor
6.2 Post Marketing Experience
The following adverse reactions have been identified during postapproval use of other formulations of glycopyrrolate for other indications. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Additional adverse reactions identified during postapproval use of glycopyrrolate tablets include: loss of taste and suppression of lactation.
7 DRUG INTERACTIONS
Drugs Affected by Reduced GI Transit Time
Glycopyrrolate reduces GI transit time, which may result in altered release of certain drugs when formulated in delayed- or controlled-release dosage forms.
- The passage of potassium chloride tablets through the GI tract may be arrested or delayed with coadministration of glycopyrrolate. Solid dosage forms of potassium chloride are contraindicated [see Contraindications (4)].
- Digoxin administered as slow dissolution oral tablets may have increased serum levels and enhanced action when administered with glycopyrrolate. Monitor patients receiving slow dissolution digoxin for increased action if glycopyrrolate oral solution is coadministered regularly. Consider the use of other oral dosage forms of digoxin (e.g., elixir or capsules).
Amantadine
The anticholinergic effects of glycopyrrolate may be increased with concomitant administration of amantadine. Consider decreasing the dose of glycopyrrolate during coadministration of amantadine.
Drugs Whose Plasma Levels May be Increased by Glycopyrrolate
Coadministration of glycopyrrolate may result in increased levels of certain drugs.
- Atenolol’s bioavailability may be increased with coadministration of glycopyrrolate. A reduction in the atenolol dose may be needed.
- Metformin plasma levels may be elevated with coadministration of glycopyrrolate, increasing metformin’s pharmacologic and toxic effects. Monitor clinical response to metformin with concomitant glycopyrrolate administration; consider a dose reduction of metformin if warranted.
Drugs Whose Plasma Levels May be Decreased by Glycopyrrolate
Coadministration of glycopyrrolate may result in decreased levels of certain drugs.
- Haloperidol’s serum level may be decreased when coadministered with glycopyrrolate, resulting in worsening of schizophrenic symptoms, and development of tardive dyskinesia. Closely monitor patients if coadministration cannot be avoided.
- Levodopa’s therapeutic effect may be reduced with glycopyrrolate administration. Consider increasing the dose of levodopa.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data in pregnant women for glycopyrrolate oral solution to inform decisions concerning any drug-associated risks. In pregnant rats, daily oral administration of glycopyrrolate during organogenesis at dose exposures 2.5 to 113 times the exposure at the maximum recommended human dose (MRHD) did not result in an increased incidence of gross external or visceral defects [ see Data]. When glycopyrrolate was administered intravenously to pregnant rabbits during organogenesis at dose exposures equivalent to up to approximately 7.8 times the exposure at the MRHD, no adverse effects on embryo-fetal development were seen. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
Glycopyrrolate was orally administered to pregnant rats at dosages of 50, 200, and 400 mg/kg/day during the period of organogenesis. These dosages resulted in systemic exposures (estimated AUC 0-inf values) approximately 2.5, 23, and 113 times, respectively, the estimated systemic exposure in humans at the MRHD (9 mg per day, administered in three divided doses). Glycopyrrolate had no effect on maternal survival, but significantly reduced mean maternal body weight gain over the period of dosing at all dosages evaluated. Mean fetal weight was significantly reduced in the 200 and 400 mg/kg/day dose groups. There were two litters with all resorbed fetuses in the 400 mg/kg/day dose group. There were no effects of treatment on the incidence of gross external or visceral defects. Minor treatment-related skeletal effects included reduced ossification of various bones in the 200 and 400 mg/kg/day dose groups; these skeletal effects were likely secondary to maternal toxicity.
Glycopyrrolate was intravenously administered to pregnant rabbits at dosages of 0.1, 0.5, and 1.0 mg/kg/day during the period of organogenesis. These dosages resulted in systemic exposures (estimated AUC 0-inf values) approximately 0.8, 4.6, and 7.8 times, respectively, the estimated systemic exposure in humans at the MRHD. Glycopyrrolate did not affect maternal survival under the conditions of this study. Mean maternal body weight gain and mean food consumption over the period of dosing were lower than the corresponding control value in the 0.5 and 1.0 mg/kg/day treatment groups. There were no effects of treatment on fetal parameters, including fetal survival, mean fetal weight, and the incidence of external, visceral, or skeletal defects.
Female rats that were pregnant or nursing were orally dosed with glycopyrrolate daily at dosages of 0, 50, 200, or 400 mg/kg/day, beginning on day 7 of gestation, and continuing until day 20 of lactation. These dosages resulted in systemic exposures (estimated AUC 0-inf values) approximately 2.5, 23, and 113 times, respectively, the estimated systemic exposure in humans at the MRHD (9 mg per day, administered in three divided doses). Mean body weight of pups in all treatment groups was reduced compared to the control group during the period of nursing, but eventually recovered to be comparable to the control group, post-weaning. No other notable delivery or litter parameters were affected by treatment in any group, including no effects on mean duration of gestation or mean numbers of live pups per litter. No treatment-related effects on survival or adverse clinical signs were observed in pups. There were no effects of maternal treatment on behavior, learning, memory, or reproductive function of pups.
8.2 Lactation
Risk Summary
There are no data on the presence of glycopyrrolate or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for glycopyrrolate oral solution and any potential adverse effects on the breastfed infant from glycopyrrolate oral solution or from the underlying maternal condition.
8.4 Pediatric Use
Glycopyrrolate oral solution was evaluated for chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling. Glycopyrrolate oral solution has not been studied in subjects under the age of 3 years.
10 OVERDOSAGE
Because glycopyrrolate is a quaternary amine which does not easily cross the blood-brain barrier, symptoms of glycopyrrolate over dosage are generally more peripheral in nature rather than central compared to other anticholinergic agents. In case of accidental overdose, therapy may include:
- Maintain an open airway, providing ventilation as necessary.
- Managing any acute conditions such as hyperthermia, coma and or seizures as applicable, and managing any jerky myoclonic movements or choreoathetosis which may lead to rhabdomyolysis in some cases of anticholinergic over dosage.
- Administering a quaternary ammonium anticholinesterase such as neostigmine to help alleviate-peripheral anticholinergic effects such as anticholinergic induced ileus.
- Administering activated charcoal orally as appropriate.
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption
In a parallel study of children (n=6 per group) aged 7-14 years undergoing intraocular surgery, subjects received either intravenous (IV) or oral glycopyrrolate as a premedication. The mean absolute bioavailability of oral glycopyrrolate tablets was low (approximately 3%) and highly variable among subjects (range 1.3 to 13.3%). A similar pattern of low and variable relative bioavailability is seen in adults.
Analysis of population pharmacokinetic data from normal adults and children with cerebral palsy associated chronic moderate to severe drooling failed to demonstrate linear pharmacokinetics across the dose range. In the same analysis, population estimates of the apparent oral clearance (scaled by weight in children and adults) ranged from 5.28 - 38.95 L/hr/kg for healthy adults and 8.07 - 25.65 L/hr/kg for patients with cerebral palsy, a reflection of the low and highly variable oral bioavailability of glycopyrrolate.
Absorption of glycopyrrolate oral solution (fasting) was compared to that of a marketed glycopyrrolate oral tablet. The Cmax after oral solution administration was 23% lower compared to tablet administration and AUC0-inf was 28% lower after oral solution administration. Mean Cmax after oral solution administration in the fasting state was 0.318 ng/mL, and mean AUC 0-24 was 1.74 ng.hr/mL. Mean time to maximum plasma concentration for glycopyrrolate oral solution was 3.1 hours, and mean plasma half-life was 3.0 hours.
In healthy adults, a high fat meal was shown to significantly affect the absorption of glycopyrrolate oral solution (10 mL, 1 mg/5 mL). The mean Cmax under fed high fat meal conditions was approximately 74% lower than the Cmax observed under fasting conditions. Similarly, mean AUC 0-T was reduced by about 78% by the high fat meal compared with the fasting AUC 0-T. A high fat meal markedly reduces the oral bioavailability of glycopyrrolate oral solution. Therefore, glycopyrrolate oral solution should be dosed at least one hour before or two hours after meals. Pharmacokinetic results (mean ± SD) are described in Table 3.
Table 3: Pharmacokinetic Parameters (mean±SD) for Glycopyrrolate Oral Solution, Fasting and Fed, in Healthy Adults C max
(ng/mL)
T max
(hrs)
AUC 0-T
(ng·hr/mL)
AUC 0-Inf
(ng·hr/mL)
T 1/2
(hrs)
Fasting (n=37)
0.318 ± 0.190
3.10 ± 1.08
1.74 ± 1.07
1.81 ± 1.09
3.0 ± 1.2
Fed (n=36)
0.084 ± 0.081
2.60 ± 1.12
0.38 ± 0.14
0.46 ± 0.13*
3.2 ± 1.1*
* n=35
Distribution
After IV administration, glycopyrrolate has a mean volume of distribution in children aged 1 to 14 years of approximately 1.3 to 1.8 L/kg, with a range from 0.7 to 3.9 L/kg. In adults aged 60-75 years, the volume of distribution was lower (0.42 L/kg +/- 0.22).
Metabolism
In adult patients who underwent surgery for cholelithiasis and were given a single IV dose of tritiated glycopyrrolate, approximately 85% of total radioactivity was excreted in urine and <5% was present in T-tube drainage of bile. In both urine and bile, >80% of the radioactivity corresponded to unchanged drug. These data suggest a small proportion of IV glycopyrrolate is excreted as one or more metabolites.
Elimination
Approximately 65-80% of an IV glycopyrrolate dose was eliminated unchanged in urine in adults. In two studies, after IV administration to pediatric patients ages 1-14 years, mean clearance values ranged from 1.01- 1.41 L/kg/hr (range 0.32 -2.22 L/kg/hr). In adults, IV clearance values were 0.54 ± 0.14 L/kg/hr.
Pediatrics
The estimated apparent clearance of glycopyrrolate from a population pharmacokinetic analysis (scaled by weight in children and adults) of oral and IV data was found to be 13.2 L/hr/kg or 92.7 L/hr for a typical 70 kg subject. In the same population based analysis, gender was not identified as having an effect on either glycopyrrolate clearance or systemic exposure.
Gender
Population pharmacokinetic evaluation of adults and children administered IV or oral glycopyrrolate identified no effect of gender on glycopyrrolate clearance or systemic exposure.
Race
The pharmacokinetics of glycopyrrolate by race has not been characterized.
Elderly
Glycopyrrolate pharmacokinetics have not been characterized in the elderly.
Renal Impairment
In one study, glycopyrrolate 4 mcg/kg was administered intravenously in uremic patients undergoing renal transplantation surgery. Mean AUC (10.6 mcg·h/L), mean plasma clearance (0.43 L/hr/kg) and mean 3-hour urinary excretion (0.7%) for glycopyrrolate were significantly different than those of control patients (3.73 µg·h/L, 1.14 L/hr/kg, and 50%, respectively). These results suggest that elimination of glycopyrrolate is severely impaired in patients with renal failure.
Hepatic Impairment
Glycopyrrolate is largely renally eliminated. The pharmacokinetics of glycopyrrolate have not been evaluated in patients with hepatic impairment
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
When glycopyrrolate was administered via oral gavage to mice for up to 24 months at dosages of 2.5, 7, and 20 mg/kg/day in both genders, resulting in systemic exposures (estimated AUC 0-inf values) approximately 0.1, 0.3, and 0.8 times, respectively, the estimated systemic exposure in humans at the MRHD (9 mg per day, administered in three divided doses), no significant changes in tumor incidence were observed when compared to control.
When glycopyrrolate was administered via oral gavage to rats for up to 24 months at dosages of 5, 15, and 40 mg/kg/day in both genders, resulting in systemic exposures approximately 0.2, 0.8, and 2 times, respectively, the estimated systemic exposure in humans at the MRHD, no significant changes in tumor incidence were observed when compared to control.
Glycopyrrolate did not elicit any genotoxic effects in the Ames mutagenicity assay, the human lymphocyte chromosome aberration assay, or the micronucleus assay.
Glycopyrrolate was assessed for effects on fertility or general reproductive function in rats. Rats of both genders received glycopyrrolate at dosages up to 100 mg/kg/day via oral gavage, resulting in systemic exposures (estimated AUC0-inf values) in males and females up to approximately 11 and 15 times, respectively, the estimated systemic exposure in humans at the MRHD. No treatment-related effects on fertility or reproductive parameters were observed in either gender in this study.
14 CLINICAL STUDIES
Glycopyrrolate oral solution was evaluated in a multi-center, randomized, double-blind, placebo-controlled, parallel, eight-week study for the control of pathologic drooling in children (Study 1). The study enrolled 38 subjects aged 3-23 years; thirty-six subjects were aged 3-16 years and two patients were greater than 16 years. The subjects were male or female, weighed at least 13 kg (27 lbs), and had cerebral palsy, mental retardation, or another neurologic condition associated with problem drooling defined as drooling in the absence of treatment so that clothing became damp on most days (approximately five to seven days per week). Subjects were randomized in a 1:1 fashion to receive glycopyrrolate oral solution or placebo. Doses of study medication were titrated over a 4-week period to optimal response beginning at 0.02 mg/kg three times a day increasing doses in increments of approximately 0.02 mg/kg three times per day every 5-7 days, not to exceed the lesser of approximately 0.1 mg/kg three times per day or 3 mg three times per day.
Subjects were evaluated on the 9-point modified Teacher’s Drooling Scale (mTDS), which is presented below. The mTDS evaluations were recorded by parents/caregivers 3 times daily approximately two hours post-dose on evaluation days during pre-treatment baseline and at Weeks 2, 4, 6 and 8 of therapy.
Modified Teacher’s Drooling Scale
1= Dry: never drools
2= Mild: only the lips are wet; occasionally
3= Mild: only the lips are wet; frequently
4= Moderate: wet on the lips and chin; occasionally 5= Moderate: wet on the lips and chin; frequently
6= Severe: drools to the extent that clothing becomes damp; occasionally
7= Severe: drools to the extent that clothing becomes damp; frequently
8= Profuse: clothing, hands, tray, and objects become wet; occasionally
9= Profuse: clothing, hands, tray, and objects become wet; frequently
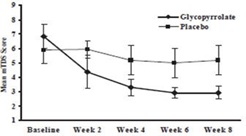
Responders were defined as subjects with at least a 3-point reduction in mean daily m TDS scores from baseline to Week 8. Table 4 presents the proportion of responders at Week 8 and Figure 1 presents the mean m TDS values from baseline through Week 8.
Table 4: Percentage of Responders at Week 8 Glycopyrrolate Oral Solution
Group (N=20)
Placebo Group (N=18)
15/20 (75%)
2/18 (11%)
Figure 1. Mean (± 2 Standard Errors) mDTS Scores
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 68022-0156-1; 1 mg/5mL clear, cherry-flavored solution; 16 oz. bottle.
Close17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
- Advise patients/caregivers to measure glycopyrrolate oral solution with an accurate measuring device. A household teaspoon is not an accurate measuring device. Patients/caregivers should use a dosing cup available in pharmacies to accurately measure the correct milliliter dose. An oral syringe, also available in pharmacies, should be used to dispense glycopyrrolate oral solution into the child’s mouth from the cup. A pharmacist can recommend an appropriate measuring device and can provide instructions for measuring the correct dose.
- Administering glycopyrrolate oral solution with a high fat meal substantially reduces the amount of glycopyrrolate absorbed. Administer glycopyrrolate oral solution at least one hour before or two hours after meals.
- Glycopyrrolate oral solution is started at a low dose and gradually titrated over a period of weeks based on therapeutic response and adverse reactions. Patients/caregivers should not increase the dose without the physician’s permission.
- Common adverse reactions from glycopyrrolate oral solution include overly dry mouth, constipation, vomiting, flushing of the skin or face, and urinary retention. Side effects can sometimes be difficult to detect in some patients with neurologic problems who cannot adequately communicate how they feel. If side effects become troublesome after increasing a dose, decrease the dose to the prior one and contact your physician.
- Constipation is the most common side effect of glycopyrrolate, and if constipation occurs, stop administering glycopyrrolate to the patient and call their healthcare practitioner.
- Inability of the patient to urinate, dry diapers or undergarments, irritability or crying may be signs of urinary retention, and if urinary retention occurs, patients/caregivers should stop administering glycopyrrolate and call their healthcare practitioner.
- If the patient develops a skin rash, hives or an allergic reaction, parents/caregivers should stop administering glycopyrrolate and call their healthcare practitioner as this could be a sign of hypersensitivity to this product.
- Drugs like glycopyrrolate can reduce sweating, and if the patient is in a hot environment and flushing of the skin occurs this may be due to overheating. Avoid exposure of the patient to hot or very warm environmental temperatures to avoid overheating and the possibility of heat exhaustion or heat stroke.
Manufactured by:
Suven Pharmaceuticals Limited
Pashamylaram, Telangana 502307, India
M.L No: 24/MD/AP/2009/F/CC
Revised: 07/2021
Lb30156-4-02
PATIENT and CAREGIVER INFORMATION
Glycopyrrolate oral solution
Please read the Patient and Caregiver Information that comes with glycopyrrolate oral solution before you start giving it to your child, and each time you get a refill. This leaflet does not take the place of talking with your doctor about your child’s medical condition or treatment.
What is glycopyrrolate oral solution?
Glycopyrrolate oral solution is a prescription medicine used in children with medical conditions that cause too much (abnormal) drooling.
Who should not take glycopyrrolate oral solution?
Do not give glycopyrrolate oral solution to anyone who:
- has problems urinating
- has a bowel problem called paralytic ileus
- lacks normal bowel tone or tension
- has severe ulcerative colitis or certain other serious bowel problems with severe ulcerative colitis
- has myasthenia gravis
What should I tell my doctor before giving glycopyrrolate oral solution to my child?
Tell your doctor if your child:
- has any allergies
- has any stomach or bowel problems, including ulcerative colitis
- has any problems with constipation
- has thyroid problems
- has high blood pressure
- has heart problems or abnormal heart beats
- has a hiatal hernia with gastroesophageal reflux disease (GERD)
- has any eye problems
- has any problems urinating
- has any other medical conditions
- is pregnant or plans to become pregnant. It is not known if glycopyrrolate oral solution can harm an unborn baby.
- is breastfeeding or plans to breastfeed. It is not known if glycopyrrolate oral solution passes into breast milk and if it can harm the baby.
Tell your doctor about all of the medicines that your child takes, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicine may affect the way glycopyrrolate oral solution works, and glycopyrrolate oral solution may affect how some other medicines work.
How should I give glycopyrrolate oral solution?
- Give glycopyrrolate oral solution exactly as prescribed by your child’s doctor.
- Give glycopyrrolate oral solution 1 hour before or 2 hours after meals.
- Your doctor will tell you how much (milliliters or mLs) of glycopyrrolate oral solution to give your child.
- Do not change the dose of glycopyrrolate oral solution unless your doctor tells you to.
- You must measure the dose of glycopyrrolate oral solution before giving it to your child. Use a special marked dose measuring cup (available at most pharmacies) to measure the right dose of glycopyrrolate oral solution.
- To help make sure that your child swallows the dose, you should use an oral syringe to give the child each dose of glycopyrrolate oral solution, after you measure the dose needed with a dose measuring cup. Oral syringes are also available at most pharmacies.
- If you have questions about how to measure the dose or how to use an oral syringe, ask your pharmacist or doctor.
- The dose of glycopyrrolate oral solution that is needed to control drooling may be different for each child. Glycopyrrolate oral solution is usually started at a low dose, and slowly increased as directed by your doctor. This slow increase in dose continues until the best dose for your child is reached, to control drooling.
- During this time it is important to stay in close contact with your child’s doctor, and tell the doctor about any side effects that your child has. See “What are the possible side effects of glycopyrrolate oral solution?"
What should I avoid while taking glycopyrrolate oral solution?
- Glycopyrrolate oral solution may cause sleepiness or blurred vision. Do not drive a car, operate heavy machinery, or do other dangerous activities while taking glycopyrrolate oral solution.
- Avoid overheating. See “What are the possible side effects of glycopyrrolate oral solution?”
What are the possible side effects of glycopyrrolate oral solution?
Glycopyrrolate oral solution can cause serious side effects including:
- Constipation. Constipation is common with glycopyrrolate oral solution. Tell your doctor if your child strains with bowel movements, goes longer between bowel movements, cannot have a bowel movement, or their stomach is firm and large. The dose of glycopyrrolate oral solution may need to be decreased or stopped.
- Diarrhea and intestinal blockage. Diarrhea can be an early symptom of a blockage in the intestine. This is especially true if your child has a colostomy or ileostomy. Tell your doctor if your child has any diarrhea while taking glycopyrrolate oral solution.
- Problems with control of body temperature (overheating or heat stroke). Glycopyrrolate oral solution can cause your child to sweat less. Your child can become overheated, and develop heat stroke if they are in an area that is very hot. Avoid overheating. Call your doctor right away if your child becomes sick and has any of these symptoms of heatstroke:
- hot, red skin
- decreased alertness or passing out (unconsciousness)
- fast, weak pulse
- fast, shallow breathing
- increased body temperature (fever)
The most common side effects of glycopyrrolate oral solution include:
- dry mouth
- vomiting
- flushing of the face or skin
- nasal congestion
- headache
- swollen sinuses (sinusitis)
- upper respiratory tract infection
- problems urinating, difficulty starting urination
Tell your doctor if your child has any side effect that concerns you or that does not go away. These are not all the possible side effects of glycopyrrolate oral solution.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store glycopyrrolate oral solution?
Store glycopyrrolate oral solution between 68°F to 77°F (20°C to 25°C).
Keep glycopyrrolate oral solution out of the reach of children.
General information about glycopyrrolate oral solution:Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use glycopyrrolate oral solution for a condition for which it was not prescribed. Do not give glycopyrrolate oral solution to other people even if they have the same condition. It may harm them.
This leaflet summarizes the most important information about glycopyrrolate oral solution. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about glycopyrrolate oral solution that is written for health professionals.
For more information, go to: safety@lambda-cro.com
What are the ingredients in glycopyrrolate oral solution?
Active Ingredient: glycopyrrolate
Inactive Ingredients: citric acid, glycerin, sour cherry flavor, ethylparaben, propylene glycol, propylparaben, saccharin sodium, sodium citrate, sorbitol solution, and purified water.
Manufactured by:
Suven Pharmaceuticals Limited
Pashamylaram, Telangana 502307, India
M.L No: 24/MD/AP/2009/F/CC
Revised: 07/2021
Lb30156-4-02
-
Glycopyrrolate oral solution 1 mg/5 mL (0.2 mg/mL)
NDC: 68022-6156-1 16 fl. oz. (473 mL) Glycopyrrolate - oral solution - 1 mg/5 mL (0.2 mg/mL) Rx Only - For Oral Use Only - Lb30156-1-01 07/2021 - Each 5 ml contains: glycopyrrolate 1 ...
NDC: 68022-6156-1 16 fl. oz.
(473 mL)
Glycopyrrolate
oral solution
1 mg/5 mL (0.2 mg/mL)
Rx Only
For Oral Use Only
Lb30156-1-01 07/2021
Each 5 ml contains: glycopyrrolate 1 mg
Usual dosage:
See Package Insert for full prescribing
information
Store between 20· - 25°C (68° - 77°F);
excursions permitted to 15° - 30°C
(59° - 86°F). [See USP Controlled Room
Temperature]Keep out ot reach of children.
Manufactured by:
Suven Pharmaceuticals Limited,
Pashamylaram, Telangana 502307, India.
ML No. 24/MD/AP/2009/F/CCLb30156-1-01 07/2021

Close
-
INGREDIENTS AND APPEARANCEProduct Information
GLYCOPYRROLATE ORAL SOLUTION glycopyrrolate oral solution liquid Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68022-0156 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 1 mg in 5 mL Product Characteristics Color Score Shape Size Flavor CHERRY (Sour Cherry) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68022-0156-1 1 in 1 CARTON 07/05/2022 1 500 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212467 07/05/2022 Labeler - Suven Pharmaceuticals Limited (677604288) Registrant - Suven Pharmaceuticals Limited (861468675)
CloseEstablishment Name Address ID/FEI Business Operations Suven Pharmaceuticals Limited 677604288 analysis(68022-0156) , api manufacture(68022-0156) , manufacture(68022-0156) , pack(68022-0156) , label(68022-0156)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
GLYCOPYRROLATE ORAL SOLUTION liquid
Number of versions: 2
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jan 18, 2024 | 5 (current) | download |
| Jul 7, 2022 | 4 | download |
Get Label RSS Feed for this Drug
GLYCOPYRROLATE ORAL SOLUTION liquid
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=78ff2314-3a87-8554-e053-2991aa0a33ea
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
GLYCOPYRROLATE ORAL SOLUTION liquid
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 68022-0156-1 |