Label: DENTAL CLEAR PREMIUM- sodium fluoride rinse
- NDC Code(s): 24765-127-01
- Packager: Pharmacal-International. Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
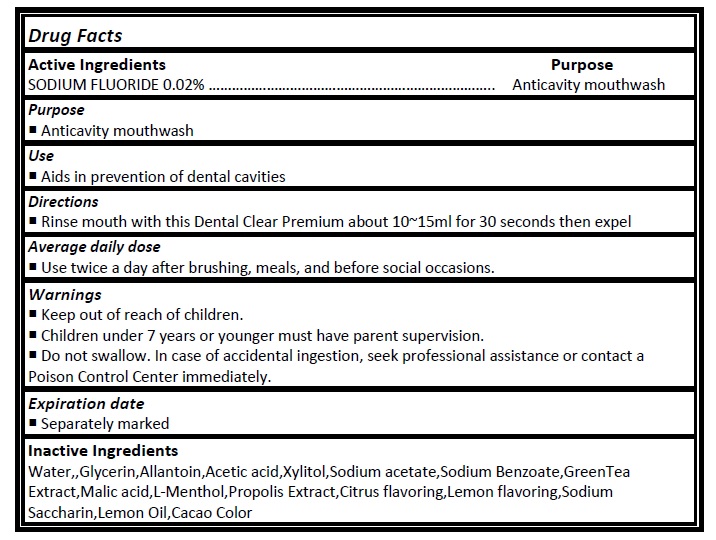
Active ingredient
Sodium Fluoride (0.02%)
Directions
- Rinse mouth with this Dental Clear Premium about 10~15ml for 30 seconds then expel
Average daily dose
- Use twice a day after brushing, meals, and before social occasions.
- Principal display panel
-
INGREDIENTS AND APPEARANCE
DENTAL CLEAR PREMIUM
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24765-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) ACETIC ACID (UNII: Q40Q9N063P) XYLITOL (UNII: VCQ006KQ1E) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM BENZOATE (UNII: OJ245FE5EU) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MALIC ACID (UNII: 817L1N4CKP) RACEMENTHOL (UNII: YS08XHA860) SACCHARIN SODIUM ANHYDROUS (UNII: I4807BK602) LEMON OIL (UNII: I9GRO824LL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24765-127-01 11 mL in 1 POUCH; Type 0: Not a Combination Product 03/14/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/14/2020 Labeler - Pharmacal-International. Co., Ltd. (557805060) Registrant - Pharmacal-International. Co., Ltd. (557805060)