Label: MORPHINE SULFATE suppository
- NDC Code(s): 63629-2535-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0574-7112
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MORPHINE SULFATE SUPPOSITORIES safely and effectively. See full prescribing information for MORPHINE SULFATE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL EXPOSURE; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Addiction, Abuse, and Misuse
Morphine sulfate suppositories expose patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing morphine sulfate suppositories, and monitor all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1)].
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with use of morphine sulfate suppositories. Monitor for respiratory depression, especially during initiation of morphine sulfate suppositories or following a dose increase [see Warnings and Precautions (5.2)].
Accidental Exposure
Accidental exposure of even one dose of morphine sulfate suppositories, especially by children, can result in a fatal overdose of morphine [see Warnings and Precautions (5.2)].
Neonatal Opioid Withdrawal Syndrome
Prolonged use of morphine sulfate suppositories during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions (5.3)].
Risks From Concomitant Use With Alcohol, Benzodiazepines Or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.4), Drug Interactions (7)].
- •
- Reserve concomitant prescribing of morphine sulfate suppositories and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- •
- Limit dosages and durations to the minimum required.
- •
- Follow patients for signs and symptoms of respiratory depression and sedation.
-
1 INDICATIONS AND USAGE Morphine sulfate suppositories are indicated for the management of acute and chronic pain severe enough to require and opioid analgesic and for which alternative treatments are ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Dosage and Administration Instructions - Morphine sulfate suppositories are available in four strengths: 5 mg per suppository, 10 mg per suppository, 20 mg per suppository, and 30 ...
-
3 DOSAGE FORMS AND STRENGTHS Morphine Sulfate Suppositories are available in four strengths: • 5 mg per suppository - • 10 mg per suppository - • 20 mg per suppository - • 30 mg per suppository
-
4 CONTRAINDICATIONS Morphine sulfate suppositories are contraindicated in patients with: • Significant respiratory depression [see Warnings and Precautions (5.2)] • Acute or severe bronchial asthma in an ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Addiction, Abuse, and Misuse - Morphine sulfate suppositories contain morphine, a Schedule II controlled substance. As an opioid, morphine sulfate suppositories expose users to the risks of ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are described, or described in greater detail, in other sections: • Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)] • Life-Threatening ...
-
7 DRUG INTERACTIONS Table 1 includes clinically significant drug interactions with morphine sulfate suppositories. Table 1: Clinically Significant Drug Interactions with Morphine Sulfate ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Prolonged use of opioid analgesics during pregnancy can cause neonatal opioid withdrawal syndrome [see Warnings and Precautions (5.3)]. There are no available data ...
-
9 DRUG ABUSE AND DEPENDENCE 9.1 Controlled Substance - Morphine sulfate suppositories contains morphine, a Schedule II controlled substance. 9.2 Abuse - Morphine sulfate suppositories contain morphine, a substance with ...
-
10 OVERDOSAGE Clinical Presentation - Acute overdose with morphine sulfate suppositories can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold ...
-
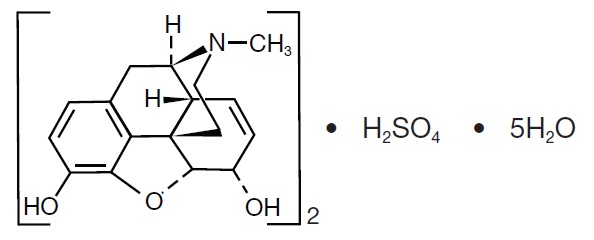
11 DESCRIPTION Morphine sulfate suppositories are an opioid agonist, available in 5 mg, 10 mg, 20 mg, and 30 mg strengths for rectal administration. The chemical name is morphinan-3,6-diol ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Morphine is a full opioid agonist and is relatively selective for the mu-opioid receptor, although it can bind to other opioid receptors at higher doses. The principal ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of morphine have not been ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Morphine Sulfate Suppositories are white to cream torpedo shaped suppositories - Strength: 10 mg - Carton Count: 12 suppositories - NDC Number: NDC 63629-2535-1 - Store at 20° to 25°C (68° to 77°F) [see ...
-
17 PATIENT COUNSELING INFORMATION Addiction, Abuse, and Misuse - Inform patients that the use of morphine sulfate suppositories, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to ...
-
Medication Guide Morphine Sulfate (mor’ feen sul’ fate) Suppositories, CII - Morphine Sulfate Suppositories are: • A strong prescription pain medicine that contains an opioid (narcotic) that is used to manage ...
-
PRINCIPAL DISPLAY PANELMorphine Sulfate 10 mg (CII) Suppos, #12
-
INGREDIENTS AND APPEARANCEProduct Information