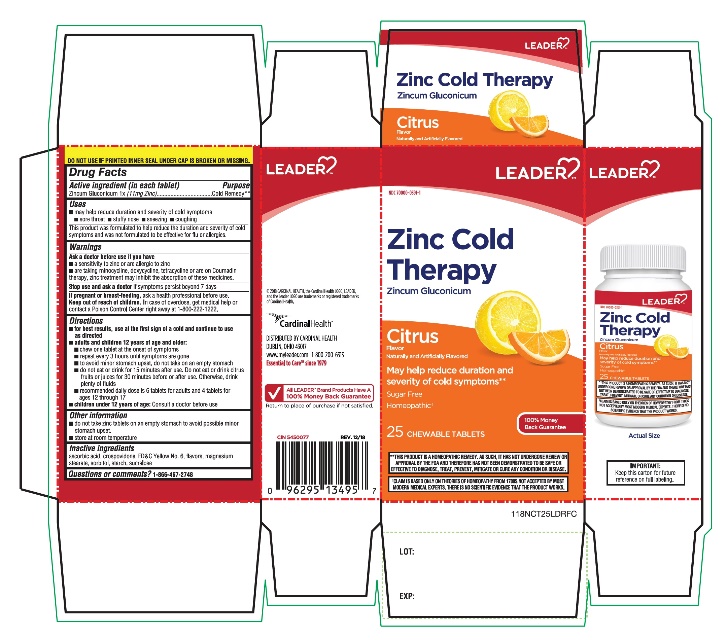
Label: ZINC COLD THERAPY- zinc gluconate tablet
- NDC Code(s): 70000-0391-1
- Packager: CARDINAL HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
ACTIVE INGREDIENTActive ingredient (in each tablet)Purpose - Cold Remedy - Zincum Gluconicum 1x(11 mg Zinc)
-
Uses▪ may help reduce duration and severity of cold symptoms - ▪ sore throat - ▪ stuffy nose - ▪ sneezing - ▪ coughing - This product was formulated to help reduce the duration and severity of cold ...
-
WarningsAsk a doctor before use if you have - ▪ a sensitivity to zinc or are allergic to zinc - ▪ are taking minocycline, doxycycline, tetracycline or are on Coumadin therapy, zinc treatment may ...
-
Directions▪ For best results, use at the first sign of a cold and continue to use as directed - ▪ adults and children 12 years of age and older: ▪ chew one tablet at the onset of symptoms - ▪ repeat every ...
-
Other information▪ do not take zinc tablets on an empty stomach to avoid possible minor stomach upset. ▪ store at room temperature
-
Inactive ingredientsascorbic acid, crospovidone, FD&C yellow # 6 lake, magnesium stearate, natural and artificial flavor sorbitol, starch, sucralose
-
Questions or comments? 1-866-467-2748
-
PRINCIPAL DISPLAY PANEL COMPARE TO ZICAM® COLD REMEDY RAPID MELTS Ionizable Zinc Content * Zinc Cold Therapy - Zincum Gluconicum - Citrus - Flavor - Naturally and Artificially Flavored - May help reduce duration and severity ...
-
INGREDIENTS AND APPEARANCEProduct Information