Label: ALBON- sulfadimethoxine tablet
- NDC Code(s): 54771-8431-1, 54771-8432-1, 54771-8433-1, 54771-8433-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 7, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
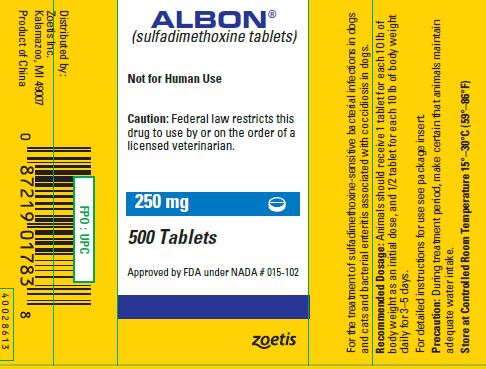
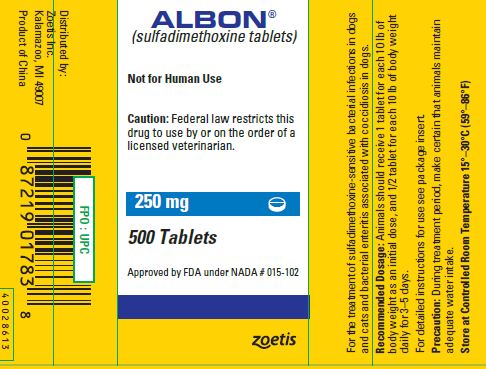
SPL UNCLASSIFIED SECTIONALBON® (sulfadimethoxine tablets) Tablets - For the treatment of sulfadimethoxine-sensitive bacterial infections in dogs and cats and bacterial enteritis associated with coccidiosis in dogs.
-
CAUTIONFederal law restricts this drug to use by or on the order of a licensed veterinarian.
-
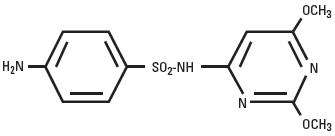
DescriptionAlbon is a low-dosage, rapidly absorbed, long-acting sulfonamide, effective for the treatment of a wide range of bacterial infections commonly encountered in dogs and cats. Sulfadimethoxine is a ...
-
INDICATIONS AND USAGEAlbon is indicated for the treatment of respiratory, genitourinary tract, enteric, and soft tissue infections in dogs and cats: tonsillitis - pharyngitis - bronchitis - pneumonia - cystitis ...
-
WARNINGNot for human use.
-
PRECAUTIONDuring treatment period, make certain that animals maintain adequate water intake. If animals show no improvement within 2 or 3 days, reevaluate your diagnosis.
-
DOSAGE AND ADMINISTRATIONInitial Dose: 25 mg/lb (55 mg/kg) of animal body weight. Subsequent Daily Doses: 12.5 mg/lb (27.5 mg/kg) of animal body weight. For ease of administration in animals of varying weights, 3 tablet ...
-
TOXICITY AND SAFETYData regarding acute and chronic toxicities of sulfadimethoxine indicate the drug is very safe. The LD50 in mice is greater than 2 g/kg of body weight when administered intraperitoneally and ...
-
STORAGEStore at controlled room temperature 15°–30°C (59°–86°F).
-
HOW SUPPLIEDAlbon Tablets are available in the following strengths for dogs and cats: 125 mg, 250 mg, or 500 mg sulfadimethoxine per tablet.
-
REFERENCES1. Data on file, Pfizer Animal Health. 2. Stowe CM: The sulfonamides. In Jones LM (ed), Veterinary Pharmacology and Therapeutics, Ames, Iowa, Iowa State University Press, chapter 33, 1965. 3 ...
-
SPL UNCLASSIFIED SECTIONApproved by FDA under NADA # 015-102 - zoetis - Distributed by: Zoetis Inc. Kalamazoo, MI 49007 - 40028552 - Revised: August 2019
-
PRINCIPAL DISPLAY PANEL - 125 mg Tablet Bottle Label

-
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle Label
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label

-
INGREDIENTS AND APPEARANCEProduct Information