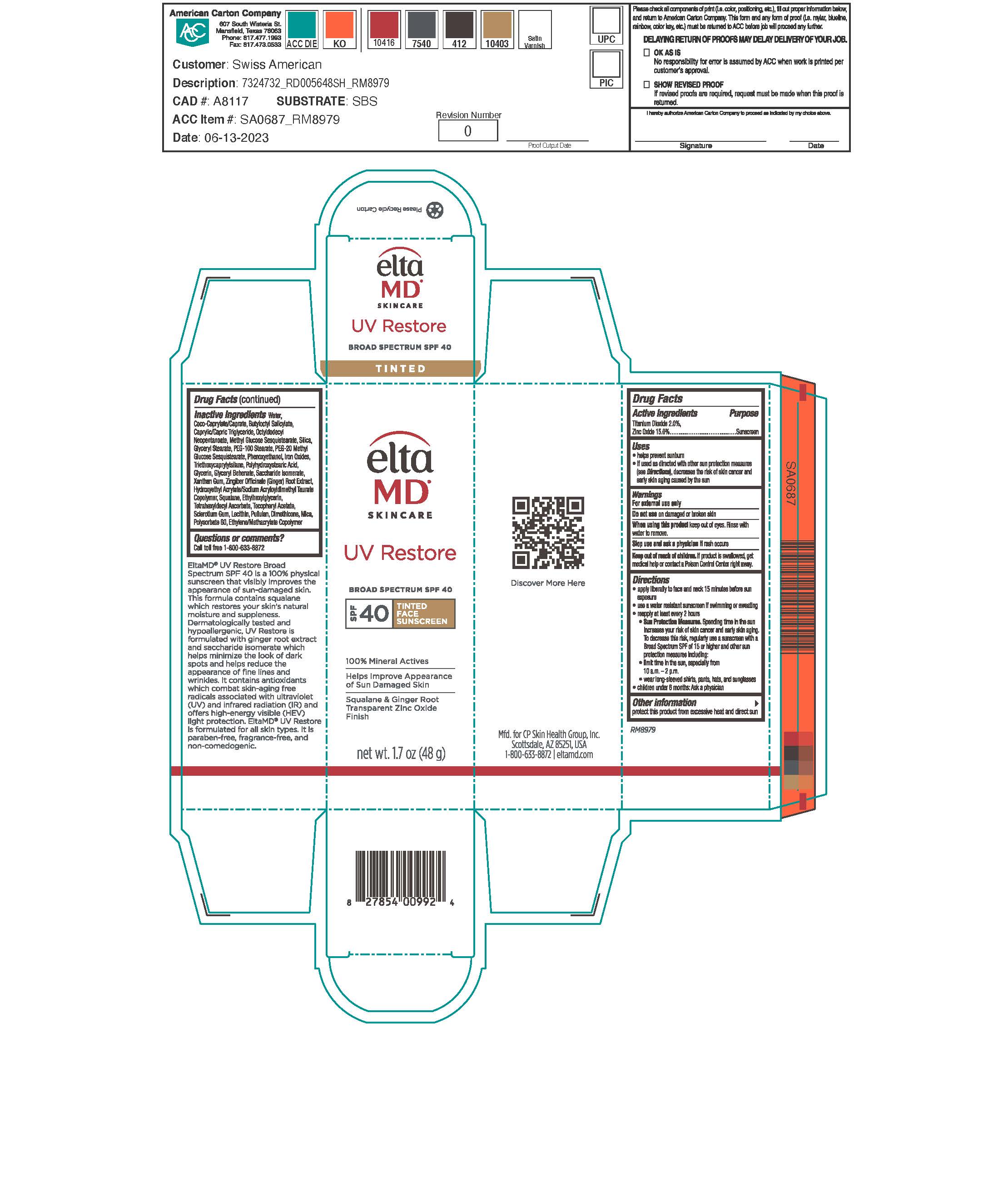
Label: ELTAMD UV RESTORE TINTED- titanium dioxide and zinc oxide sunscreen lotion
- NDC Code(s): 72043-2640-1, 72043-2640-2, 72043-2640-3, 72043-2640-4
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Active Ingredients
- Keep out of reach of children
- Uses
- Uses
-
Directions
Apply liberally 15 minutes before sun exposure. Use a water-resistant sunscreen if swimming or sweating. Reapply at least every 2 hours. Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 am to 2 pm. Wear long-sleeve shirts, pants, hats, and sunglasses. Children under 6 months: ask a physician.
- Other Information
-
Inactive Ingredients
water, coco-caprylate/caprate, butyloctyl salicylate, octyldodecyl neopentanoate, caprylic/capric triglyceride, methyl glucose sesquistearate, silica, glyceryl stearate, PEG-100 stearate, PEG-20 methyl glycose sesquisterate, phenoxyethanol, triethoxycaprylylsilane, polyhydroxystearic acid, glycerin, saccharide isomerate, glyceryl behenate, xanthan gum, zingiber officiale (ginger) root extract, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, squalene, ethylhexylglycerin, tocopheryl acetate, sclerotium gum, lecithin, pulluan, dimethicone, polysorbate 60, ethylene/methacrylate copolymer, mica, iron oxides
- Questions?
- Labeling
-
INGREDIENTS AND APPEARANCE
ELTAMD UV RESTORE TINTED
titanium dioxide and zinc oxide sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72043-2640 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 20 g in 1000 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 150 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) METHYL GLUCOSE SESQUISTEARATE (UNII: V1YW10H14D) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-100 STEARATE (UNII: YD01N1999R) PEG-20 METHYL GLUCOSE SESQUISTEARATE (UNII: 0345752X7U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIDE ISOMERATE (UNII: W8K377W98I) XANTHAN GUM (UNII: TTV12P4NEE) ZINGIBER CASSUMUNAR ROOT (UNII: 3NG517NPD8) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) GLYCERYL MONOBEHENATE (UNII: A626UU0W2A) SQUALANE (UNII: GW89575KF9) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) BETASIZOFIRAN (UNII: 2X51AD1X3T) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PULLULAN (UNII: 8ZQ0AYU1TT) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 60 (UNII: CAL22UVI4M) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72043-2640-2 57 g in 1 TUBE; Type 0: Not a Combination Product 09/15/2020 2 NDC:72043-2640-4 2 g in 1 PACKET; Type 0: Not a Combination Product 09/15/2020 3 NDC:72043-2640-1 14 g in 1 BOTTLE; Type 0: Not a Combination Product 07/07/2022 4 NDC:72043-2640-3 48 g in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/15/2020 Labeler - CP Skin Health Group, Inc. (611921669) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(72043-2640)