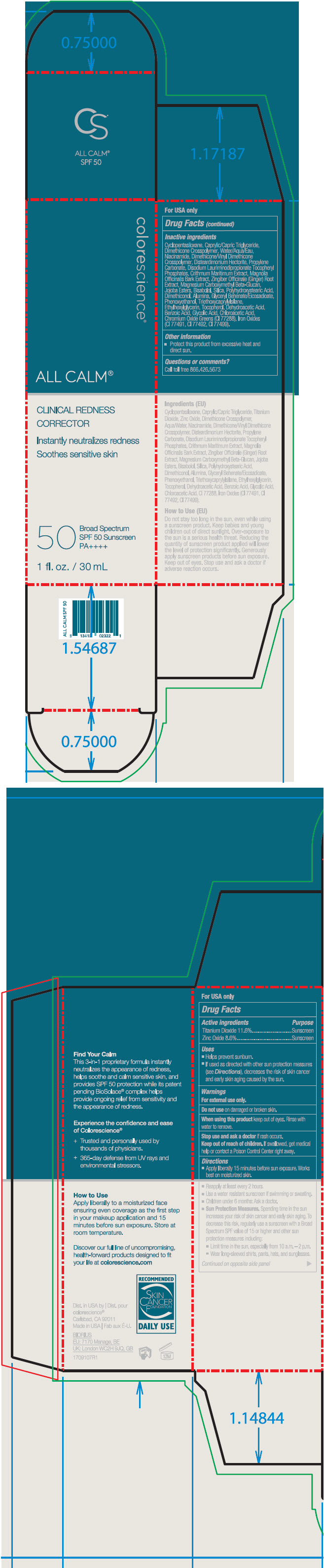
Label: ALL CALM CLINICAL REDNESS CORRECTOR SPF 50- titanium dioxide and zinc oxide liquid
- NDC Code(s): 68078-023-02, 68078-023-24
- Packager: Colorescience
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
ACTIVE INGREDIENTActive ingredientsPurpose - Titanium Dioxide 11.6%Sunscreen - Zinc Oxide 8.6%Sunscreen
-
UsesHelps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
-
WarningsFor external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash ...
-
DirectionsApply liberally 15 minutes before sun exposure. Works best on moisturized skin. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Children under 6 months ...
-
Inactive ingredientsCyclopentasiloxane, Caprylic/Capric Triglyceride, Water/Aqua/Eau, Dimethicone Crosspolymer, Niacinamide, Disteardimonium Hectorite, Dimethicone/Vinyl Dimethicone Crosspolymer, Propylene Carbonate ...
-
Other informationProtect this product from excessive heat and direct sun.
-
Questions or comments?Call toll free 866.426.5673
-
SPL UNCLASSIFIED SECTIONDist. in USA by - colorescience® Carlsbad, CA 92011
-
PRINCIPAL DISPLAY PANEL - 30 mL Tube Cartoncolorescience® ALL CALM™ CLINICAL REDNESS - CORRECTOR - Instantly neutralizes redness - Soothes sensitive skin - 50 - Broad Spectrum - SPF 50 Sunscreen - PA++++ 1 fl. oz. / 30 mL
-
INGREDIENTS AND APPEARANCEProduct Information