Label: AKLIEF- trifarotene cream
- NDC Code(s): 0299-5935-02, 0299-5935-30, 0299-5935-45, 0299-5935-75
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AKLIEF ®Cream safely and effectively. See full prescribing information for AKLIEF Cream.
AKLIEF(trifarotene) cream, for topical use
Initial U.S. Approval: 2019INDICATIONS AND USAGE
AKLIEF Cream is a retinoid indicated for the topical treatment of acne vulgaris in patients 9 years of age and older. ( 1)
DOSAGE AND ADMINISTRATION
- For topical use only. Not for oral, ophthalmic or intravaginal use.
- Apply a thin layer of AKLIEF Cream to the affected areas of the face and/or trunk once a day, in the evening, on clean and dry skin. Avoid contact with the eyes, lips, paranasal creases, and mucous membranes. ( 2)
DOSAGE FORMS AND STRENGTHS
Cream: 0.005% trifarotene. ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
- Skin irritation: Erythema, scaling, dryness, and stinging/burning may be experienced with use of AKLIEF Cream. Use a moisturizer from the initiation of treatment, and, if appropriate, reduce the frequency of application of AKLIEF Cream, suspend or discontinue use. ( 5.1)
- Ultraviolet Light and Environmental Exposure: Minimize exposure to sunlight and sunlamps. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided. ( 5.2)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 1%) in patients treated with AKLIEF Cream were application site irritation, application site pruritus, and sunburn ( 6).
To report SUSPECTED ADVERSE REACTIONS, contact Galderma Laboratories, L.P. at 1-866-735-4137 or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Skin Irritation
5.2 Ultraviolet Light and Environmental Exposure
6 ADVERSE REACTIONS
6.1 Clinical trials experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply a thin layer of AKLIEF Cream to the affected areas once daily, in the evening, on clean and dry skin.
- One pump actuation should be enough to cover the face (i.e., forehead, cheeks, nose, and chin).
- Two actuations of the pump should be enough to cover the upper trunk (i.e., reachable upper back, shoulders and chest). One additional pump actuation may be used for middle and lower back if acne is present.
The use of a moisturizer is recommended as frequently as needed from the initiation of treatment.
Avoid contact with the eyes, lips, paranasal creases, mucous membranes.
AKLIEF Cream is for topical use only. Not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Skin Irritation
Patients using AKLIEF Cream may experience erythema, scaling, dryness, and stinging/burning. Maximum severity of these reactions typically occurred within the first 4 weeks of treatment, and severity decreased with continued use of the medication. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of application of AKLIEF Cream, or suspend use temporarily. If severe reactions persist the treatment may be discontinued.
Avoid application of AKLIEF Cream to cuts, abrasions, or eczematous or sunburned skin. Use of “waxing” as a depilatory method should be avoided on skin treated with AKLIEF Cream.
5.2 Ultraviolet Light and Environmental Exposure
Minimize unprotected exposure to ultraviolet rays (including sunlight and sunlamps) during treatment with AKLIEF Cream. Warn patients who normally experience high levels of sun exposure and those with inherent sensitivity to sun to exercise caution. Use of sunscreen products and protective clothing over treated areas is recommended when exposure cannot be avoided.
-
6 ADVERSE REACTIONS
6.1 Clinical trials experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect rates observed in practice. In the three Phase 3 clinical trials, 1673 subjects with acne vulgaris on the face and trunk, 9 years and older were exposed to AKLIEF Cream. Of these, 1220 subjects were treated once daily for up to 12 weeks and 453 were treated once daily for up to 1 year.
Adverse reactions reported in the 2 randomized, double-blind, vehicle-controlled 12-week clinical trials in ≥1.0% of subjects treated with AKLIEF Cream (and for which the rate exceeded the rate for vehicle), as well as the corresponding rates reported in subjects treated with the vehicle cream are presented in Table 1.
Table 1. Adverse Reactions Occurring in ≥ 1.0% of Subjects with Acne Vulgaris of the Face and Trunk in the Two 12-week Phase 3 Clinical Trials Preferred Term AKLIEF Cream
(N=1220)Vehicle
Cream
(N=1200)Application site irritation 91 (7.5) 4 (0.3) Application site pruritus 29 (2.4) 10 (0.8) Sunburn 32 (2.6) 6 (0.5) Additional adverse reactions that were reported in more than one subject treated with AKLIEF Cream (and at a frequency <1%) included application site pain, application site dryness, application site discoloration, application site rash, application site swelling, application site erosion, acne, dermatitis allergic, and erythema.
In the one-year, open-label safety trial that included 453 subjects 9 years and older, with acne vulgaris of the face and trunk, the pattern of adverse reactions for AKLIEF Cream was similar to that experienced in the 12-week controlled trials. A total of 12.6% of subjects had at least one adverse reaction during the trial, and 2.9% of subjects had an adverse reaction leading to treatment discontinuation. The most common adverse reactions (≥1% of subjects) for the entire trial were application site pruritus (4.6%), application site irritation (4.2%), and sunburn (5.5%). The frequency of adverse reactions decreased over time.
Skin irritation was evaluated by active assessment of erythema, scaling, dryness, and stinging/burning and collected separately. In the two 12-week Phase 3 clinical trials, these signs/symptoms were assessed at baseline and at least one post-baseline visit, in 1214 subjects (for face) and 1202 subjects (for trunk) treated with AKLIEF Cream. The percentage of subjects who were assessed to have these signs and symptoms at any post baseline visit and at a severity worse than baseline are summarized in Table 2.
Table 2. Application Site Tolerability Reactions at Any Post Baseline Visit Face AKLIEF Cream
N=1214
Maximum Severity during TreatmentVehicle Cream
N=1194
Maximum Severity during TreatmentMild Moderate Severe Mild Moderate Severe Erythema 30.6% 28.4% 6.2% 21% 6.8% 0.8% Scaling 37.5% 27.1% 4.9% 23.7% 5.9% 0.3% Dryness 39% 29.7% 4.8% 29.9% 6.8% 0.8% Stinging/Burning 35.6% 20.6% 5.9% 15.9% 3.8% 0.5% Trunk N=1202 N=1185 Erythema 26.5% 18.9% 5.2% 12.7% 4.4% 0.4% Scaling 29.7% 13.7% 1.7% 13.2% 2.6% 0.1% Dryness 32.9% 16.1% 1.8% 17.8% 3.9% 0.1% Stinging/Burning 26.1% 10.9% 4.3% 9.2% 2.2% 0.5% Local tolerability on the face in subjects treated with AKLIEF Cream worsened for any of the signs/symptoms compared with baseline to a score of moderate for up to 30% of subjects, or severe for up to 6% of subjects. On the trunk, the corresponding percentages were up to 19% (moderate) and up to 5% (severe). The scores reached maximum severity at Week 1 for the face, and at Week 2 to 4 of treatment for the trunk, and decreased thereafter.
In the open-label, 1-year Phase 3 trial, the local tolerability profile was comparable to that observed in the two pivotal Phase 3 trials.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from clinical trials with AKLIEF Cream use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are case reports of major birth defects similar to those seen in fetuses exposed to oral retinoids in pregnant women exposed to other topical retinoids, but these case reports do not establish a pattern or association with retinoid-related embryopathy.
In animal reproduction studies, oral doses of trifarotene administered to pregnant rats and rabbits during organogenesis that resulted in systemic exposures more than 800 times the systemic exposure at the maximum recommended human dose (MRHD) of AKLIEF Cream resulted in adverse fetal effects, including fetal deaths and external, visceral, and skeletal malformations (see Data). The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Oral administration of trifarotene to pregnant rats during the period of organogenesis at doses that resulted in systemic exposures greater than 1600 times those in humans at the MRHD of AKLIEF Cream resulted in adverse fetal effects, including fetal deaths, reduced mean fetal weight, and external, visceral, and skeletal malformations.
Oral administration of trifarotene to pregnant rabbits during the period of organogenesis at doses that resulted in systemic exposures at least 800 times those in humans at the MRHD of AKLIEF Cream resulted in adverse fetal effects, including defects of the tail, limbs, urogenital organs, and vertebral column.
Trifarotene administered orally to female rats from gestation Day 6 to lactation Day 20, at doses that resulted in systemic exposures up to 594 times those in humans at the MRHD of AKLIEF Cream, had no effect on maternal function or behavior, including gestation, delivery, pup-rearing, lactation and nursing, or survival or development of pups .There were no effects of maternal treatment on behavior, learning, memory, or reproductive function of pups.
8.2 Lactation
Risk Summary
There are no data on the presence of trifarotene in human milk, the effects on the breastfed infant, or the effects on milk production. In animal studies, trifarotene was present in rat milk with oral administration of the drug. When a drug is present in animal milk, it is likely that the drug will be present in human milk. It is possible that topical administration of large amounts of trifarotene could result in sufficient systemic absorption to produce detectable quantities in human milk (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AKLIEF Cream and any potential adverse effects on the breastfed infant from AKLIEF Cream or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breastmilk, use AKLIEF Cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply AKLIEF Cream directly to the nipple and areola to avoid direct infant exposure.
8.4 Pediatric Use
Safety and effectiveness of AKLIEF Cream for the topical treatment of acne vulgaris have been established in pediatric patients age 9 years to 17 years based on evidence from well-controlled clinical trials, a long-term safety trial, and a pharmacokinetic trial. A total of 897 pediatric subjects aged 9 to 17 years received AKLIEF Cream in the clinical trials [see Clinical Pharmacology ( 12.3) and Clinical Studies ( 14)] .
Safety and effectiveness of AKLIEF Cream have not been established in pediatric subjects under the age of 9 years. -
11 DESCRIPTION
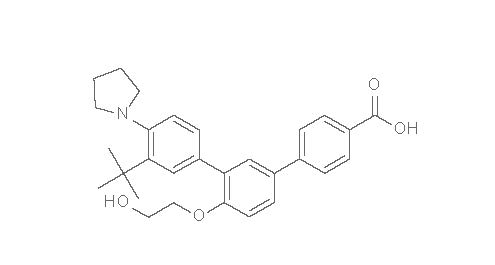
AKLIEF Cream for topical administration contains 0.005% (50 mcg/g) trifarotene. Trifarotene is a terphenyl acid derivative and is a retinoid. The chemical name of trifarotene is 3”-tert-Butyl-4’-(2-hydroxy-ethoxy)-4”-pyrrolidin-1-yl-[1,1’,3’,1”]terphenyl-4- carboxylic acid. Trifarotene has the molecular formula of C 29H 33NO 4, the molecular weight of 459.58, and the following structural formula:
Trifarotene is a white to off-white to slightly yellow powder with the melting point of 245°C. It is practically insoluble in water with pKa1 of 5.69 and pKa2 of 4.55.
AKLIEF (trifarotene) Cream 0.005% contains the following inactive ingredients: allantoin, copolymer of acrylamide and sodium acryloyldimethyltaurate dispersion 40% in isohexadecane, cyclomethicone, 95% (v/v) ethanol, medium-chain triglycerides, phenoxyethanol, propylene glycol, purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Trifarotene is an agonist of retinoic acid receptors (RAR), with particular activity at the gamma subtype of RAR. Stimulation of RAR results in modulation of target genes which are associated with various processes, including cell differentiation and mediation of inflammation. The exact process by which trifarotene ameliorates acne is unknown.
12.2 Pharmacodynamics
At the approved recommended dosage, AKLIEF Cream does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Pharmacokinetics of trifarotene was evaluated in a study involving 19 adult subjects with acne vulgaris following once daily application of AKLIEF Cream for 29 days (daily dose range 1.5 g/day to 2 g/day) to the face, shoulders, chest and upper back.
Absorption:
Systemic concentrations were at steady state following 2 weeks of treatment and were quantifiable in 7 subjects. Steady state C maxranged from below the limit of quantification (less than 5 pg/mL) to 10 pg/mL and AUC 0-24hranged from 75 to 104 pg.h/mL in adults. No drug accumulation is expected with long-term use.
Distribution
Plasma protein binding is approximately 99.9%
Elimination
The terminal half-life ranged from 2 to 9 hours.
Metabolism
Trifarotene is primarily metabolized by CYP2C9, CYP3A4, CYP2C8, and to a lesser extent by CYP2B6 in vitro.
Excretion
Trifarotene is primarily excreted by the feces.
Specific Populations
Pediatric Patients
Steady state C maxranged from less than 5 pg/mL to 9 pg/mL and AUC 0-24hranged from 89 to 106 pg.h/mL in pediatrics (10 to 17- years-old). Steady state conditions were achieved in patients following 2 weeks of topical administration. No drug accumulation is expected with long-term use.
Drug Interactions Studies
Clinical Studies and Model-Based Approaches
No clinically significant differences in the pharmacokinetics of trifarotene were predicted when used concomitantly with fluconazole (a moderate CYP2C9 and CYP3A inhibitor).In Vitro Studies
Cytochrome P450 (CYP) Enzymes: AKLIEF Cream is not expected to inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4, or induce CYP1A2, 2B6, and 3A4.
Transporter Systems: AKLIEF Cream is not expected to inhibit MATE, OATP, OAT, OCT, BCRP, P-gp, BSEP, or MRP. -
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Trifarotene was not carcinogenic when topically applied to mice daily for up to 24 months in the vehicle of the product (AKLIEF Cream) at concentrations of 0.0005% or 0.001% w/w. The systemic exposures at the highest doses evaluated in mice were approximately 82 (males) and 99 (females) times higher than the human exposure at the MRHD of AKLIEF Cream.
Trifarotene was not carcinogenic when administered orally to rats daily for up to 24 months at doses up to 0.75 mg/kg/day in males and 0.2 mg/kg/day in females. The systemic exposures at the highest doses evaluated in rats were approximately 645 (males) and 1642 (females) times higher than the human exposure at the MRHD of AKLIEF Cream.
Trifarotene was negative in an in vitro bacterial reverse mutation (Ames) assay, an in vitro micronucleus assay in primary human lymphocytes, an in vitro mouse lymphoma assay with L5178Y/TK +/-cells, and an in vivo micronucleus assay in rats.
Trifarotene was assessed for effects on fertility or general reproductive function in rats. Males received trifarotene via oral gavage for 4 weeks prior to mating, during mating, and up to scheduled termination (approximately 6 weeks in total), and females were treated via oral gavage for 2 weeks prior to mating through Day 7 of gestation. No adverse effects on fertility or reproductive parameters, including sperm motility and concentration, were observed at the highest doses evaluated, which resulted in systemic exposures approximately 1755 (males) and 1726 (females) times higher than the human exposure at the MRHD of AKLIEF Cream.
-
14 CLINICAL STUDIES
AKLIEF Cream applied once daily in the evening was evaluated in the treatment of moderate facial and truncal acne vulgaris in two randomized, multicenter, parallel group, double-blind, vehicle-controlled trials of identical design, Study 1 (NCT02566369) and Study 2 (NCT02556788). The trials were conducted in a total of 2420 subjects aged 9 years and older, who were treated for up to 12 weeks with either AKLIEF Cream (1214 subjects) or vehicle cream (1206 subjects). Subjects were encouraged to use a moisturizer as desired, while allowing an interval of approximately 1 hour before or after the study treatment application.
Acne severity was evaluated using a 5-point Investigator’s Global Assessment (IGA) scale for the face and a 5-point Physician’s Global Assessment (PGA) scale for the trunk with moderate acne vulgaris defined as a score of 3. Overall, 87% of subjects were Caucasian and 55% were female. Thirty-four (1.4%) subjects were 9 to 11 years of age, 1128 (47%) subjects were 12 to 17 years of age, and 1258 (52%) subjects were 18 years and older. All subjects had moderate acne vulgaris on the face and 99% of subjects had moderate acne vulgaris on the trunk. At baseline, subjects had between 7 and 200 (average 36) inflammatory lesions on the face and between 0 and 220 (average 38) on the trunk. Additionally, subjects had 21 to 305 (average 52) non-inflammatory lesions on the face and 0 to 260 (average 46) on the trunk.
Success on the IGA/PGA scale was defined as achieving a score of 0 (clear) or 1 (almost clear) and at least a 2-grade improvement from baseline. The co-primary endpoints (evaluated on the face) were the percentage of subjects achieving success on the IGA scale, the mean absolute change in facial inflammatory lesion count from baseline, and the mean absolute change in facial non-inflammatory lesion count from baseline, all evaluated at Week 12. The co-secondary endpoints (evaluated on the trunk) were the percentage of subjects achieving success on the PGA scale, the mean absolute change in truncal inflammatory lesion count from baseline, and the mean absolute change in truncal non-inflammatory lesion count from baseline, all evaluated at Week 12. Efficacy results for acne on the face and trunk after 12 weeks of treatment are presented in Tables 3 and 4 respectively.
Table 3. Acne of the Face Efficacy Results at Week 12 (Intent-to-Treat; Multiple Imputation) - *
- Means presented in table are Least Square (LS) means
Study 1 Study 2 AKLIEF Cream Vehicle Cream AKLIEF Cream Vehicle Cream (N=612) (N=596) (N=602) (N=610) IGA Success
At least a 2-grade improvement and "Clear" (0) or
"Almost Clear" (1)29.4% 19.5% 42.3% 25.7% Inflammatory Lesions
Mean * Absolute (Percent) Change from Baseline-19.0 (-54.4%) -15.4 (-44.8%) -24.2 (-66.2%) -18.7 (-51.2%) Non-inflammatory Lesions
Mean *Absolute (Percent) Change from Baseline-25.0 (-49.7%) -17.9 (-35.7%) -30.1 (-57.7%) -21.6 (-43.9%) Table 4. Acne of the Trunk Efficacy Results at Week 12 (Intent-to-Teat on the Trunk; Multiple Imputation) - *
- Means presented in table are Least Square (LS) means
Study 1 Study 2 AKLIEF Cream Vehicle Cream AKLIEF Cream Vehicle Cream (N=600) (N=585) (N=598) (N=609) PGA Success
At least a 2-grade improvement and "Clear" (0) or "Almost Clear" (1)35.7% 25.0% 42.6% 29.9% Inflammatory Lesions
Mean * Absolute (Percent) Change from Baseline-21.4 (-57.4%) -18.8 (-50.0%) -25.5 (-65.4%) -19.8 (-51.1%) Non-inflammatory Lesions
Mean *Absolute (Percent) Change from Baseline-21.9 (-49.1%) -17.8 (-40.3%) -25.9 (-55.2%) -20.8 (-45.1%) -
16 HOW SUPPLIED/STORAGE AND HANDLING
AKLIEF Cream, 0.005% is provided as a white cream supplied in the following packaging configurations with corresponding NDC numbers:
- 45-gram pump NDC 0299-5935-45
Storage and Handling
- Store at 20˚ to 25˚C (68˚ to 77˚F); excursions permitted to 15° to 30°C (59° to 86°F)..
- Keep away from heat.
- Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Advise the patient to:
- Cleanse the area to be treated; pat dry. Apply AKLIEF Cream as a thin layer once daily in the evening to the face, avoiding the eyes, lips, nasolabial folds, and mucous membranes. A thin layer of AKLIEF Cream may also be applied to the chest, shoulders, and back.
- Avoid applying AKLIEF Cream to damaged skin (such as cuts, abrasions), eczematous areas, and sunburned skin.
- Reduce the risk of such irritation, use a moisturizer from the start of treatment, and, if appropriate, reduce the frequency of application of AKLIEF Cream or suspend use temporarily. AKLIEF Cream may cause irritation such as erythema, scaling, dryness, and stinging or burning.
- Minimize exposure to sunlight, including sunlamps and phototherapy devices.
- Use sunscreen products and protective apparel (e.g., hat) over treated areas when exposure to sunlight cannot be avoided.
- Avoid concomitant use of other potentially irritating topical products (medicated or not).
- Use AKLIEF Cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply AKLIEF Cream directly to the nipple and areola to avoid direct infant exposure.
Marketed by:
GALDERMA LABORATORIES, L.P.
Dallas, Texas 75201 USA
Made in Canada
All trademarks are the property of their respective owners.Patient Information
AKLIEF® (trifarotene) creamImportant:AKLIEF Cream is for use on the skin only. Do not use AKLIEF Cream in your mouth eyes, or vagina.
What is AKLIEF Cream?
AKLIEF Cream is a prescription medicine used on the skin (topical) to treat acne vulgaris in people 9 years of age and older.
It is not known if AKLIEF Cream is safe and effective in children younger than 9 years old.Before using AKLIEF Cream, tell your healthcare provider about all of your medical conditions, including if you :
- have skin problems, including eczema, cuts or sunburn
- are pregnant or planning to become pregnant. It is not known if AKLIEF Cream can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if AKLIEF Cream passes into your breast milk. Breastfeeding women should use AKLIEF Cream on the smallest area of skin and for the shortest time needed while breastfeeding.
Do not apply AKIEF Cream to the nipple and areola to avoid contact with your baby.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-couter medicines, vitamins and herbal supplements.
Especially tell your healthcare provider if you use any other medicine for acne.How should I use AKLIEF Cream?
- Use AKLIEF Cream exactly as your healthcare provider tell you to use it. Apply a thin layer of AKLIEF Cream over the affected areas 1 time each day, in the evening.
Applying AKLIEF Cream:
-
Wash the area where the cream will be applied and pat dry.
- If you receive a sample tube of AKLIEF Cream, follow your healthcare provider’s instructions about how much to apply.
- AKLIEF Cream comes in a pump.
- Press down on (depress) the pump 1 time to dispense a small amount of AKLIEF Cream and spread a thin layer over your face (forehead, cheeks, nose, and chin). Avoid contact with your eyes, lips, mouth, and the corners of your nose.
- Press down on the pump 2 times to dispense enough AKLIEF Cream to apply a thin layer to cover your upper trunk (the area of your upper back that you can reach, shoulders, and chest). One more pump may be used to apply a thin layer of AKLIEF Cream to your middle and lower back, if acne is present.
- When you begin treatment with AKLIEF Cream, you should begin applying a moisturizer on your skin as often as needed. See “Local skin irritation”below.
What should I avoid while using AKLIEF Cream?
- Minimize exposure to sunlight. You should avoid using sunlamps, tanning beds, and ultraviolet light during treatment with AKLIEF Cream. If you have to be in sunlight or are sensitive to sunlight, use a sunscreen with a SPF (sun protection factor) of 15 or more, and wear protective clothing and a wide-brimmed hat to cover the treated areas.
- Avoid using AKLIEF Cream on skin areas with cuts, abrasions, eczema, or on sunburned skin.
- Avoid using skin products that may dry or irritate your skin, such as:
- medicated or abrasive soaps and cleansers
- soaps, cleansers, and cosmetics that have strong skin drying effects
- products that contain high amounts of alcohol
- Avoid the use of “waxing” as a hair removal method on skin treated with AKLIEF Cream.
What are the possible side effects of AKLIEF Cream?
AKLIEF Cream may cause serious side effects including:-
Local skin irritation.Local skin reactions are common with AKLIEF Cream, are most likely to happen during the first 4 weeks of treatment and may decrease with continued use of AKLIEF Cream. Signs and symptoms of local skin reactions include:
- redness
- scaling
- dryness
- stinging or burning
To help reduce your risk of developing these local skin reactions, when you begin treatment with AKLIEF Cream, you should begin applying a moisturizer on your skin as often as needed.
Tell your healthcare provider if you develop symptoms of a local skin reaction. Your doctor may tell you to use AKLIEF Cream less often, or temporarily, or permanently stop your treatment with AKLIEF Cream.The most common side effects of AKLIEF Cream include: itching and sunburn. See “What should I avoid while usingAKLIEF Cream.
These are not all the possible side effects of AKLIEF Cream. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to GALDERMA LABORATORIES, L.P. at 1-866-735-4137.How should I store AKLIEF Cream?
- Store AKLIEF Cream at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F)
- Keep AKLIEF Cream away from heat.
- If you receive a sample tube of AKLIEF Cream from your healthcare provider, keep the tube tightly closed.
Keep AKLIEF Cream and all medicines out of the reach of children.
General information about the safe and effective use of AKLIEF Cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use AKLIEF Cream for a condition for which it was not prescribed. Do not give AKLIEF Cream to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about AKLIEF Cream that is written for health professionals.What are the ingredients in AKLIEF Cream?
Active ingredient: trifarotene
Inactive ingredients:allantoin, copolymer of acrylamide and sodium acryloyldimethyltaurate dispersion 40% in isohexadecane, cyclomethicone, 95% (v/v) ethanol, medium-chain triglycerides, phenoxyethanol, propylene glycol, purified water.
Marketed by: GALDERMA LABORATORIES, L.P., Dallas, Texas 75201 USA
All trademarks are the property of their respective owners.
Made in Canada
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 10/2023
P54485-3
- PATIENT PACKAGE INSERT
-
PACKAGE LABEL 45g CARTON
RX only
NDC 0299-5935-45
AKLIEF
(trifarotene)
Cream, 0.005%
PUMP
For topical use only
NET WT.
45 g
GALDERMA
For topical use only
Not for oral, ophthalmic or intravaginal use
USUAL DOSAGE:
Apply a thin layer to affected areas of the face and / or trunk once a day. See package insert for complete prescribing information.
EACH GRAM CONTAINS:
Active:trifarotene 0.005%
Inactive:allantoin, copolymer of acrylamide and sodium acryloyldimethyltaurate dispersion 40% in isohexadecane, cyclomethicone, 95% (v/v) ethanol, medium-chain triglycerides, phenoxyethanol, propylene glycol, purified water. Contains 5% alcohol
STORAGE:
Store at 20º to 25ºC (68º to 77ºF); excursions permitted between 15º and 30ºC (59º to 86ºF). See carton closure for lot number and expiration.
Marketed by:
GALDERMA LABORATORIES, L.P.
Dallas, TX 75201 USA
Made in Canada
All trademarks are the property of their respective owners.
P54484-1
NET WT. 45 g
-
INGREDIENTS AND APPEARANCE
AKLIEF
trifarotene creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0299-5935 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIFAROTENE (UNII: 0J8RN2W0HK) (TRIFAROTENE - UNII:0J8RN2W0HK) TRIFAROTENE 50 ug in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ACRYLIC ACID/SODIUM ACRYLATE COPOLYMER (1:1; 600 MPA.S AT 0.2%) (UNII: M4PPW69Y4H) ISOHEXADECANE (UNII: 918X1OUF1E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALCOHOL (UNII: 3K9958V90M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-5935-02 1 in 1 BLISTER PACK 10/04/2019 1 2 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0299-5935-30 1 in 1 CARTON 10/04/2019 10/05/2019 2 30 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 3 NDC:0299-5935-45 1 in 1 CARTON 10/04/2019 3 45 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:0299-5935-75 1 in 1 CARTON 10/04/2019 10/05/2019 4 75 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211527 10/04/2019 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(0299-5935)