Label: SAFE-GUARD AQUASOL- fenbendazole suspension
- NDC Code(s): 0061-1433-01, 0061-1433-02, 0061-1433-03
- Packager: Merck Sharp & Dohme Corp.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION:
- INDICATIONS:
-
DOSAGE AND ADMINISTRATION:
Safe-Guard® AquaSol must be administered orally to chickens via the drinking water at a daily dose of 1 mg/kg BW (0.454 mg/lb) for 5 consecutive days. The medicated water must be prepared daily prior to each administration. Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
-
GENERAL MIXING DIRECTIONS:
Step 1: Determine the estimated total weight of the entire group of chickens to be treated. One 3mL vial of Safe-Guard® Aquasol will treat a flock with a total body weight of up to 264 lbs for 5 consecutive days. The 3 mL presentation of Safe-Guard® AquaSol should NOT be used for flocks with a total body weight of less than 22 lbs because accurate doses cannot be measured for these flocks. Step 2: Consult the table below to determine the volume of water required to prepare the medicated water based on flock weight.
Use a clean mixing container appropriate for the daily volume of water, fill with the required amount of water, and set aside.If the total flock weight is: Measure the following volume of water: If the total flock weight is: Measure the following volume of water: 22 to 55 lbs 4 cups (0.9 L) 441 to 550 lbs 2.5 gal (9.5 L) 56 to 110 lbs 8 cups (1.9 L) 551 to 660 lbs 3 gal (11.4 L) 111 to 220 lbs 1 gal (3.8 L) 661 to 770 lbs 3.5 gal (13.2 L) 221 to 330 lbs 1.5 gal (5.7 L) 771 to 880 lbs 4 gal (15.1 L) 331 to 440 lbs 2 gal (7.6 L) Step 3: Consult the table below to determine the amount of Safe-Guard® AquaSol needed and prepare your medicated water following the mixing instructions below the table. The table below provides information for one day. The total treatment period is five consecutive days.
For each day (for 5 consecutive days) If the total flock weight is: Extract the following volume of Safe-Guard® AquaSol from the vial: And dilute into the following volume of drinking water: Less than 22 lbs Not recommended Not applicable 22 lbs 0.05 mL 4 cups (0.9 L) 23 to 33 lbs 0.075 mL 34 to 44 lbs 0.1 mL 45 to 55 lbs 0.125 mL 56 to 66 lbs 0.15 mL 8 cups (1.9 L) 67 to 77 lbs 0.175 mL 78 to 88 lbs 0.2 mL 89 to 99 lbs 0.225 mL 100 to 110 lbs 0.25 mL 111 to 121 lbs 0.275 mL 1 gal (3.7 L) 122 to 132 lbs 0.3 mL 133 to 143 lbs 0.325 mL 144 to 154 lbs 0.35 mL 155 to 165 lbs 0.375 mL 166 to 176 lbs 0.4 mL 177 to 187 lbs 0.425 mL 188 to 198 lbs 0.45 mL 199 to 209 lbs 0.475 mL 210 to 220 lbs 0.5 mL 221 to 231 lbs 0.525 mL 1.5 gal (5.6 L) 232 to 242 lbs 0.55 mL 243 to 253 lbs 0.575 mL 254 to 264 lbs 0.6 mL 265 to 275 lbs 0.625 mL 276 to 286 lbs 0.65 mL 287 to 297 lbs 0.675 mL 298 to 308 lbs 0.7 mL 309 to 319 lbs 0.725 mL 320 to 330 lbs 0.75 mL 331 to 341 lbs 0.775 mL 2 gal (7.5 L) 342 to 352 lbs 0.8 mL 353 to 363 lbs 0.825 mL 364 to 374 lbs 0.85 mL 375 to 385 lbs 0.875 mL 386 to 396 lbs 0.9 mL 397 to 407 lbs 0.925 mL 408 to 418 lbs 0.95 mL 419 to 429 lbs 0.975 mL 430 to 440 lbs 1 mL 441 to 451 lbs 1.05 mL 2.5 gal (9.4 L) 452 to 462 lbs 463 to 473 lbs 1.075 mL 474 to 484 lbs 1.1 mL 485 to 495 lbs 1.125 mL 496 to 506 lbs 1.15 mL 507 to 517 lbs 1.175 mL 518 to 528 lbs 1.2 mL 529 to 539 lbs 1.225 mL 540 to 550 lbs 1.25 mL 551 to 561 lbs 1.275 mL 3 gal (11.3 L) 562 to 572 lbs 1.3 mL 573 to 583 lbs 1.325 mL 584 to 594 lbs 1.35 mL 595 to 605 lbs 1.375 mL 606 to 616 lbs 1.4 mL 617 to 627 lbs 1.425 mL 628 to 638 lbs 1.45 mL 639 to 649 lbs 1.475 mL 650 to 660 lbs 1.5 mL 661 to 671 lbs 1.525 mL 3.5 gal (13.2 L) 672 to 682 lbs 1.55 mL 683 to 693 lbs 1.575 mL 694 to 704 lbs 1.6 mL 705 to 715 lbs 1.625 mL 716 to 726 lbs 1.65 mL 727 to 737 lbs 1.675 mL 738 to 748 lbs 1.7 mL 749 to 759 lbs 1.725 mL 760 to 770 lbs 1.75 mL 771 to 781 lbs 1.775 mL 4 gal (15.1 L) 782 to 792 lbs 1.8 mL 793 to 803 lbs 1.825 mL 804 to 814 lbs 1.85 mL 815 to 825 lbs 1.875 mL 826 to 836 lbs 1.9 mL 837 to 847 lbs 1.925 mL 848 to 858 lbs 1.95 mL 859 to 869 lbs 1.975 mL 870 to 880 lbs 2 mL 
Agitate the vial containing Safe-Guard® AquaSol (fenbendazole oral suspension) for approximately 10 seconds. 
Attach the provided syringe to the vial cap while keeping the container upright. Use only the syringe supplied by Merck Animal Health for the preparation of the medicated water. 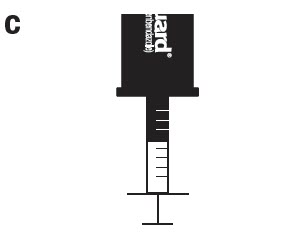
Leave the syringe attached to the vial and turn both upside down. Hold the syringe and vial firmly in one hand. With your free hand, pull the plunger to withdraw the correct amount of Safe-Guard® AquaSol into the syringe. Before you remove the syringe out of the vial, check the syringe for air bubbles. If there are air bubbles in the syringe, proceed to Step d. If there are no air bubbles in the syringe, proceed to Step e. 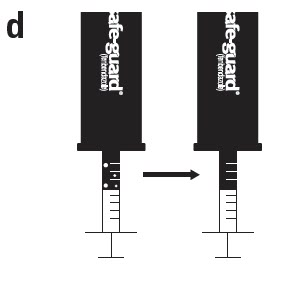
If bubbles are in the syringe, hold the syringe and vial upside down and tap the side of the syringe until the bubbles float to the top. Push the bubbles out with the plunger back into the vial. If needed, draw more Safe-Guard® AquaSol until you have the correct dose. Confirm that there are no air bubbles in the syringe before proceeding to Step e. 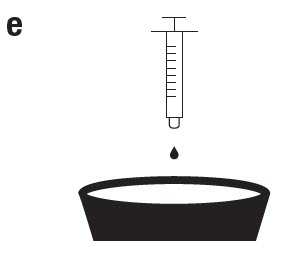
Detach the syringe from the cap and fully dispense its contents into the mixing container that contains the measured volume of drinking water. Do not submerge or allow the syringe to come in contact with the water. Mix well by stirring until the medicated water appears uniformly hazy. Each treatment day, empty all the flocks' waterers before adding the medicated water. Provide this medicated water to your birds first thing in the morning, when it is peak of consumption. If you have more than one waterer, distribute the total volume of medicated water in equal parts among the waterers. During treatment, all birds must have unrestricted access to the medicated water, and no access to other sources of water. After complete consumption of the medicated water, the animals should have access to non-medicated drinking water ad libitum. Ensure that the total amount of medicated water offered is consumed. -
CLEANING INSTRUCTIONS FOR DOSING SYRINGE:
- a:
- Do not discard the syringe as it will be needed for next treatment. The provided syringe can be reused. Perform maintenance in a clean, sanitary work area free from potential contaminants, and discard all water used to clean the syringe as waste.
- b:
- Clean all external debris off the syringe with hot tap water.
- c:
- Rinse the syringe in clean tap water by pulling and pushing the plunger a few times before disassembling the syringe.
- d:
- Disassemble the syringe, which includes the syringe barrel and the plastic plunger, and clean with hot tap water.
- i.
- When you disassemble the syringe to clean it, check that the syringe has worked properly.
If product has accumulated in the syringe barrel or on the plastic plunger, discard the syringe and use a new syringe. - ii.
- If the plastic plunger sticks when inserted back into the syringe barrel, discard the syringe and use a new syringe.
Do not use lubricant on the plastic plunger to reduce sticking.
To obtain replacement syringes, contact Merck Animal Health Customer Care at 1-800-521-5767.
- e:
- Reassemble the syringe once all parts of the syringe are dry.
- f:
- Store the syringe in a clean, dry location.
-
USER SAFETY WARNINGS:
Not for use in humans. Keep out of reach of children. Protective gloves should be used and care should be taken when handling the product to avoid skin and eye exposure and accidental ingestion. Accidental exposure may result in skin and eye irritation. Accidental ingestion may cause gastrointestinal disturbances and hypersensitivity reactions in humans. Safety Data Sheets (SDS's) can be found at https://www.msd.com/research/safety-data-sheets/# or by calling 1-800-521-5767.
- WITHDRAWAL PERIODS:
-
OTHER WARNINGS:
Parasite resistance may develop to any dewormer. All dewormers require accurate dosing for best results. Following the use of any dewormer, effectiveness of treatment should be monitored. A decrease of effectiveness over time may indicate the development of resistance to the dewormer administered. The parasite management plan should be adjusted accordingly based on regular monitoring.
-
EFFECTIVENESS:
Six pivotal dose confirmation studies and five field effectiveness studies were conducted to evaluate the effectiveness of Safe-Guard® AquaSol oral suspension against adult A. galli in broiler chickens and replacement chickens and against A. galli and H. gallinarum in breeding chickens and laying hens. Safe-Guard® AquaSol was administered orally in drinking water at 1 mg fenbendazole/kg body weight/day for 5 consecutive days. The chickens were necropsied 7 to 8 days after the last treatment, and adult worms in the intestines and ceca of the chickens in the control and treated groups were counted to determine percent efficacy.
Three dose confirmation studies were conducted in European Union (EU), using 105 Rhode Island Red breed hens (2 years old) for each study. In all three studies, the efficacy against A. galli (97.9%, 97.3%, and 93.9%) and H. gallinarum (99.8%, 96.9%, and 97.3%) was greater than 90%. A fourth dose confirmation study was conducted in the United States (US) using 264 Rhode Island Red breed hens (12 months old). In the study, the efficacy against adult A. galli and H. gallinarum was 98.7% and 99.2%, respectively. A fifth dose confirmation study was conducted in the US using 176 Cobb breed broiler chickens (4 to 5 weeks old). In the study, the efficacy against adult A. galli was 99.4%.
A sixth dose confirmation study was conducted in the US using 176 Ross breed broiler chickens (4 to 5 weeks old). In the study, the efficacy against adult A. galli was 100%.
A field effectiveness study was conducted in the EU in a flock of 13,244 Hy-Line layer breed replacement chickens (13 weeks old). Fifteen chickens were necropsied before treatment initiation, and 15 chickens were necropsied seven days after treatment for worm counts. The efficacy against adult A. galli was 90.2%. A second field effectiveness study was conducted in the US using 550 Ross breed broiler chickens (4 to 5 weeks old). The efficacy against adult A. galli was 100%. A third field effectiveness study was conducted in the US using 550White Leghorn replacement chickens (14 weeks old). The efficacy against adult A. galli and H. gallinarum was 100% and 88.9%, respectively. A fourth field effectiveness study was conducted in the US using 550 Cobb breed breeder hens (63 weeks old). The efficacy against adult A. galli and H. gallinarum was 97.6% and 95.3%, respectively. A fifth effectiveness study was conducted in the US using 550 Cobb breed broiler chickens (4 to 5 weeks old). The efficacy against adult A. galli was 100%.
The pivotal dose confirmation studies and field effectiveness studies demonstrated substantial evidence of effectiveness of Safe-Guard® AquaSol at the dose of 1 mg fenbendazole/kg body weight/day for 5 consecutive days against adult A. galli in broiler chickens and replacement chickens and against adult A. galli and H. gallinarum in breeding chickens and laying hens.
-
ANIMAL SAFETY:
Two margin of safety studies (growing broiler chickens and laying hens at peak egg production) and one reproductive safety study (broiler breeder chickens) were conducted. These studies supported the safety of Safe-Guard® AquaSol in broiler chickens, replacement chickens, laying hens and breeding chickens when administered in drinking water at 1 mg fenbendazole/kg body weight/ day for 5 consecutive days.
The margin of safety in broiler chickens was conducted in 480 broiler chickens. Safe-Guard® AquaSol was administered orally as medicated drinking water to three groups of 120 chickens (60 male and 60 female in each group) at 1, 3, and 5 mg fenbendazole/kg body weight/day (1, 3, and 5 times the recommended label dose) for 15 consecutive days (3 times the recommended duration). Another group of 120 chickens (60 male and 60 female) was provided non-medicated drinking water and used as a control group. In all chickens, feed and water intake, body weights, clinical health, and mortality were recorded. Hematology and clinical chemistry parameters were evaluated in 24 chickens from each group. At the end of the treatment phase, gross necropsies were performed on 48 chickens from each group, and organs weights were evaluated. Histopathologic examinations were performed on 48 chickens each from the control and 5 mg fenbendazole/kg body weight groups. No clinically significant effects related to the administration of Safe-Guard® AquaSol were observed for any of the parameters evaluated.
The margin of safety in laying hens was conducted in 144 laying hens. Safe-Guard® AquaSol was administered orally as medicated drinking water to three groups of 36 hens at 1, 3, and 5 times the recommended label dose (1, 3, and 5 mg fenbendazole/kg body weight/day) for 15 consecutive days (3 times the recommended duration). Another group of 36 hens was provided non-medicated drinking water and used as control group. In all hens, feed and water intake, body weights, clinical health, mortality, egg production, and egg quality parameters (including egg shell thickness and strength, egg weight, and Haugh unit) were evaluated. Hematology and clinical chemistry parameters were evaluated in 12 hens from each group.
At the end of the treatment phase, gross necropsies were performed on 12 hens from each group, and organs weights were evaluated. Histopathologic examinations were performed on 12 hens each from the control and 5 mg fenbendazole/kg body weight groups. No clinically significant effects related to the administration of Safe-Guard® AquaSol were observed for any of the parameters evaluated.
The reproductive safety in broiler breeding chickens was conducted in 220 broiler breeder chickens.
Safe-Guard® AquaSol was administered orally as medicated drinking water to a group of 110 breeding chickens (10 male and 100 female) at 3 mg fenbendazole/kg body weight/day (3 times the recommended label dose) for 21 consecutive days (4 times the recommended duration). Another group of 110 breeding chickens (10 male and 100 female) were provided non-medicated drinking water and used as a control group. The parameters evaluated in the study included feed and water intake, body weights, clinical health, egg production and weight, fertility, hatchability, and 14-day old chick viability. Necropsy of unhatched eggs was performed to record the percentage of dead embryos and dead and culled hatchlings. At the end of the treatment phase, 30 breeding chickens (10 male and 20 female) from each group were necropsied; and gross pathology and weights of testes and female reproductive tracts were evaluated. Histopathologic evaluations were performed on the gross lesions collected during the necropsy. No clinically significant effects related to the administration of Safe-Guard® AquaSol were observed for any of the parameters evaluated.
-
QUESTIONS/COMMENTS?
For technical information or to report a suspected adverse event, please contact Merck Animal Health at 1-800-211-3573 or www.merck-animal-health-usa.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or http://www.fda.gov/reportanimalae
- STORAGE INFORMATION:
- HOW SUPPLIED:
- FOR PATENT INFORMATION:
- DISTRIBUTED BY:
-
PRINCIPAL DISPLAY PANEL - 3,785 mL Container Label
Approved by FDA under NADA # 141-449
1 Gallon (3,785 mL)safe-guard® AquaSol
(fenbendazole oral suspension)Suspension Concentrate, Antiparasitic
200 mg of fenbendazole/mL
For oral administration via drinking waterDESCRIPTION: Safe-Guard® AquaSol is a suspension concentrate containing
fenbendazole, an antiparasitic. Each mL of Safe-Guard® AquaSol contains
200 mg of fenbendazole.INDICATIONS:
Chickens: Safe-Guard® AquaSol is indicated for the treatment and control of
adult Ascaridia galli in broiler chickens and replacement chickens and for the
treatment and control of adult A. galli and Heterakis gallinarum in breeding
chickens and laying hens.Swine: Safe-Guard® AquaSol is indicated for swine, except for nursing piglets,
for the treatment and control of: Lungworms: Adult Metastrongylus apri, Adult
Metastrongylus pudendotectus: Gastrointestinal worms: Adult and larvae
(L3, L4 stages, liver, lung, intestinal forms) large roundworms (Ascaris suum);
Adult nodular worms (Oesophagostomum dentatum, O. quadrispinulatum);
Adult small stomach worms (Hyostrongylus rubidus); Adult and larvae (L2, L3,
L4 stages - intestinal mucosal forms) whipworms (Trichuris suis), and Kidney
worms: Adult and larvae Stephanurus dentatusDOSAGE AND ADMINISTRATION: Chickens: Safe-Guard® AquaSol
must be administered orally to chickens via the drinking water at a daily dose
of 1 mg/kg BW (0.454 mg/lb) for 5 consecutive days. Swine: Safe-Guard®
AquaSol must be administered orally to swine via the drinking water at a daily
dose of 2.2 mg/kg BW (1mg/lb) given on 3 consecutive days.Consult your veterinarian for assistance in the diagnosis, treatment, and
control of parasitism.RESIDUE WARNING: Chickens: No withdrawal period is required when used
according to labeling. Swine: Swine intended for human consumption must not be
slaughtered within 2 days from the last treatment.STORAGE INFORMATION: Store at room temperature 30°C (86°F). Once
opened, do not store the container above 25°C (77°F). Do not freeze. Use
within 6 months after opening. Use the medicated water within 24 hours.
The container was opened (write the date here): _____________See attached Product Information Insert for complete directions and
warnings before using.For customer service, adverse effects reporting, and/or a copy of the SDS,
call 1-800-211-3573.Restricted Drug (California) - Use Only as Directed.
Not for use in humans.
Distributed by: Intervet, Inc., 126 E.
Lincoln Ave. Rahway, NJ 07065.
Fenbendazole (active ingred.) made in:
see imprint. Formulated in France.Net volume 1 Gallon
MERCK
Animal Health
714042 R2
-
PRINCIPAL DISPLAY PANEL - 1,000 mL Container Label
Approved by FDA under NADA # 141-449
1,000 mL (33.8 fl oz)safe-guard® AquaSol
(fenbendazole oral suspension)Suspension Concentrate, Antiparasitic
200 mg of fenbendazole/mL
For oral administration via drinking waterDESCRIPTION: Safe-Guard® AquaSol is a suspension concentrate containing fenbendazole,
an antiparasitic. Each mL of Safe-Guard® AquaSol contains 200 mg of fenbendazole.INDICATIONS:
Chickens: Safe-Guard® AquaSol is indicated for the treatment and control of adult Ascaridia galli
in broiler chickens and replacement chickens and for the treatment and control of adult A. galli and
Heterakis gallinarum in breeding chickens and laying hens.Swine: Safe-Guard® AquaSol is indicated for swine, except for nursing piglets, for the
treatment and control of: Lungworms: Adult Metastrongylus apri, Adult Metastrongylus
pudendotectus: Gastrointestinal worms: Adult and larvae (L3, L4 stages, liver, lung, intestinal
forms) large roundworms (Ascaris summ); Adult nodular worms (Oesophagostomum dentatum,
O. quadrispinulatum); Adult small stomach worms (Hyostrongylus rubidus); Adult and larvae (L2, L3,
L4 stages - intestinal mucosal forms) whipworms (Trichuris suis), and Kidney worms: Adult and
larvae Stephanurus dentatusDOSAGE AND ADMINISTRATION: Chickens: Safe-Guard® AquaSol must be administered
orally to chickens via the drinking water at a daily dose of 1 mg/kg BW (0.454 mg/lb) for
5 consecutive days. Swine: Safe-Guard® AquaSol must be administered orally to swine via the
drinking water at a daily dose of 2.2 mg/kg BW (1 mg/lb) for 3 consecutive days.Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
RESIDUE WARNING: Chickens: No withdrawal period is required when used according
to labeling. Swine: Swine intended for human consumption must not be slaughtered within
2 days from the last treatment.STORAGE INFORMATION: Store at room temperature 30°C (86°F). Once opened, do not store the
container above 25°C (77°F). Do not freeze. Use within 6 months after opening.
Use the medicated water within 24 hours.
The container was opened (write the date here): _________________See attached Product Information Insert for complete directions and warnings before using.
For customer service, adverse effects reporting, and/or a copy of the SDS, call 1-800-211-3573.Restricted Drug (California) - Use Only as Directed.
Not for use in humans.
Distributed by: Intervet, Inc., 126 E. Lincoln Ave. Rahway, NJ 07065.
Fenbendazole (active ingred.) made in: see imprint. Formulated in France.
Net volume 1 LiterMERCK
Animal Health
714383 R2
- PRINCIPAL DISPLAY PANEL - 3 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
SAFE-GUARD AQUASOL
fenbendazole suspensionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:0061-1433 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENBENDAZOLE (UNII: 621BVT9M36) (FENBENDAZOLE - UNII:621BVT9M36) FENBENDAZOLE 200 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-1433-02 3785 mL in 1 CONTAINER 2 NDC:0061-1433-01 1000 mL in 1 CONTAINER 3 NDC:0061-1433-03 3 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141449 11/20/2015 Labeler - Merck Sharp & Dohme Corp. (001317601)




