Label: ADRENALIN (EPINEPHRINE IN SODIUM CHLORIDE)- epinephrine in sodium chloride injection
- NDC Code(s): 42023-315-01, 42023-315-10
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ADRENALIN safely and effectively. See full prescribing information for ADRENALIN.
ADRENALIN (epinephrine in sodium chloride injection), for intravenous use
Initial U.S. Approval: 1939
INDICATIONS AND USAGE
Adrenalin® is a non-selective alpha and beta adrenergic agonist indicated to:
- Increase mean arterial blood pressure in adult patients with hypotension associated with septic shock (1.1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Injection: 250 mL single-dose container with: (3)
- 2 mg epinephrine (8 mcg/mL) in 0.9% sodium chloride
- 4 mg epinephrine (16 mcg/mL) in 0.9% sodium chloride
- 5 mg epinephrine (20 mcg/mL) in 0.9% sodium chloride
- 8 mg epinephrine (32 mcg/mL) in 0.9% sodium chloride
- 10 mg epinephrine (40 mcg/mL) in 0.9% sodium chloride
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Monitor blood pressure frequently (5.1)
- Increases cardiac output and causes peripheral vasoconstriction (5.2)
- May induce cardiac arrhythmias and myocardial ischemia. (5.3)
- Avoid extravasation into tissues, which can cause local necrosis (5.4)
- May aggravate angina pectoris or produce ventricular arrhythmias (5.5)
- Constricts Renal blood vessels which may result in oliguria or renal impairment (5.5)
ADVERSE REACTIONS
Most common adverse reactions to systemically administered epinephrine are headache; anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; peripheral coldness; nausea/vomiting; and/or respiratory difficulties. Arrhythmias, including fatal ventricular fibrillation, rapid rises in blood pressure producing cerebral hemorrhage, and angina have occurred. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs that counter the pressor effects of epinephrine include alpha blockers, vasodilators such as nitrates, diuretics, antihypertensives and ergot alkaloids. (7.1)
- Drugs that potentiate the effects of epinephrine include sympathomimetics, beta blockers, tricyclic antidepressants, MAO inhibitors, COMT inhibitors, clonidine, doxapram, oxytocin, levothyroxine sodium, and certain antihistamines. (7.2)
- Drugs that increase the arrhythmogenic potential of epinephrine include beta blockers, cyclopropane and halogenated hydrocarbon anesthetics, antihistamines, exogenous thyroid hormones, diuretics, cardiac glycosides and quinidine. Observe for development of cardiac arrhythmias. (7.3)
- Potassium-depleting drugs, including corticosteroids, diuretics, and theophylline, potentiate the hypokalemic effects of epinephrine. (7.4)
USE IN SPECIFIC POPULATIONS
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
1.1. Hypotension associated with Septic Shock
2. DOSAGE AND ADMINISTRATION
2.1. General Considerations
2.2. Hypotension associated with Septic Shock
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1. Hypertension
5.2. Pulmonary Edema
5.3. Cardiac Arrhythmias and Ischemia
5.4. Extravasation and Tissue Necrosis with Intravenous Infusion
5.5. Renal Impairment
6. ADVERSE REACTIONS
7. DRUG INTERACTIONS
7.1. Drugs Antagonizing Pressor Effects of Epinephrine
7.2. Drugs Potentiating Pressor Effects of Epinephrine
7.3. Drugs Potentiating Arrhythmogenic Effects of Epinephrine
7.4. Drugs Potentiating Hypokalemic Effects of Epinephrine
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
8.2. Lactation
8.4. Pediatric Use
8.5. Geriatric Use
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
12.2. Pharmacodynamics
12.3. Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
16. HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
2.1. General Considerations
Administration
Adrenalin is a ready to administer product that requires no further dilution prior to infusion. Inspect visually for particulate matter and discoloration prior to administration; solution should be clear and colorless. Do not use if the solution is colored or cloudy, or if it contains particulate matter.
Do not open the aluminum overwrap until time of use. The premixed, ready-to-use infusion bag has a single port for insertion of the infusion set only. This port should not be used to remove content from the bag or add another medication. Once the infusion bag has been connected to the infusion set, it is stable for 24 hours, as long as the bag stays connected to the infusion set. Single dose only.
Discontinuation
When discontinuing the infusion, reduce the flow rate gradually. Avoid abrupt withdrawal. Discard unused portion.
2.2. Hypotension associated with Septic Shock
Whenever possible, give infusions of epinephrine into a large vein. Avoid using a catheter tie-in technique, because the obstruction to blood flow around the tubing may cause stasis and increased local concentration of the drug. Avoid the veins of the leg in elderly patients or in those suffering from occlusive vascular diseases.
To provide hemodynamic support in septic shock associated hypotension in adult patients, the suggested dosing infusion rate of intravenously administered epinephrine is 0.05 mcg/kg/min to 2 mcg/kg/min and is titrated to achieve a desired mean arterial pressure (MAP). The dosage may be adjusted periodically, such as every 10 to 15 minutes, in increments of 0.05 mcg/kg/min to 0.2 mcg/kg/min, to achieve the desired blood pressure goal.
After hemodynamic stabilization, wean incrementally over time, such as by decreasing doses of epinephrine every 10 minutes to determine if the patient can tolerate gradual withdrawal.
- 3. DOSAGE FORMS AND STRENGTHS
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1. Hypertension
Because individual response to epinephrine may vary significantly, monitor blood pressure frequently and titrate to avoid excessive increases in blood pressure.
Patients receiving monoamine oxidase inhibitors (MAOI) or antidepressants of the triptyline or imipramine types may experience severe, prolonged hypertension when given epinephrine.
5.2. Pulmonary Edema
Epinephrine increases cardiac output and causes peripheral vasoconstriction, which may result in pulmonary edema.
5.3. Cardiac Arrhythmias and Ischemia
Epinephrine may induce cardiac arrhythmias and myocardial ischemia in patients, especially patients suffering from coronary artery disease, or cardiomyopathy.
5.4. Extravasation and Tissue Necrosis with Intravenous Infusion
Avoid extravasation of epinephrine into the tissues, to prevent local necrosis. When Adrenalin is administered intravenously, check the infusion site frequently for free flow. Blanching along the course of the infused vein, sometimes without obvious extravasation, may be attributed to vasa vasorum constriction with increased permeability of the vein wall, permitting some leakage. This also may progress on rare occasions to superficial slough. Hence, if blanching occurs, consider changing the infusion site at intervals to allow the effects of local vasoconstriction to subside.
There is potential for gangrene in a lower extremity when infusions of catecholamine are given in an ankle vein.
Antidote for Extravasation Ischemia: To prevent sloughing and necrosis in areas in which extravasation has taken place, infiltrate the area with 10 mL to 15 mL of saline solution containing from 5 mg to 10 mg of phentolamine, an adrenergic blocking agent. Use a syringe with a fine hypodermic needle, with the solution being infiltrated liberally throughout the area, which is easily identified by its cold, hard, and pallid appearance. Sympathetic blockade with phentolamine causes immediate and conspicuous local hyperemic changes if the area is infiltrated within 12 hours.
-
6. ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in labeling:
- Hypertension [see Warnings and Precautions (5.1)]
- Pulmonary Edema [see Warnings and Precautions (5.2)]
- Cardiac Arrhythmias and Ischemia [see Warnings and Precautions (5.3)]
- Extravasation and Tissue Necrosis with Intravenous Infusion [see Warnings and Precautions (5.4)]
- Renal Impairment [see Warnings and Precautions (5.5)]
The following adverse reactions associated with the infusion of epinephrine were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Cardiovascular disorders: tachycardia, supraventricular tachycardia, ventricular arrhythmias, myocardial ischemia, myocardial infarction, limb ischemia, pulmonary edema
Gastrointestinal disorders: Nausea, vomiting
General disorders and administrative site conditions: Chest pain, extravasation
Metabolic: hypoglycemia, hyperglycemia, insulin resistance, hypokalemia, lactic acidosis
Nervous system disorders: Headache, nervousness, paresthesia, tremor, stroke, central nervous system bleeding
Psychiatric disorders: Excitability
Renal disorders: Renal insufficiency
Respiratory: Pulmonary edema, rales
Skin and subcutaneous tissue disorders: Diaphoresis, pallor, piloerection, skin blanching, skin necrosis with extravasation
-
7. DRUG INTERACTIONS
7.1. Drugs Antagonizing Pressor Effects of Epinephrine
- α-blockers, such as phentolamine
- Vasodilators, such as nitrates
- Diuretics
- Antihypertensives
- Ergot alkaloids
- Phenothiazine antipsychotics
7.2. Drugs Potentiating Pressor Effects of Epinephrine
- Sympathomimetics
- β-blockers, such as propranolol
- Tricyclic anti-depressants
- Monoamine oxidase (MAO) inhibitors
- Catechol-O-methyl transferase (COMT) inhibitors, such as entacapone
- Clonidine
- Doxapram
- Oxytocin
7.3. Drugs Potentiating Arrhythmogenic Effects of Epinephrine
Patients who are concomitantly receiving any of the following drugs should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions (5.5) and Adverse Reactions (6)].
- β-blockers, such as propranolol
- Cyclopropane or halogenated hydrocarbon anesthetics, such as halothane
- Antihistamines
- Thyroid hormones
- Diuretics
- Cardiac glycosides, such as digitalis glycosides
- Quinidine
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Risk Summary
Limited published data on epinephrine use in pregnant women are not sufficient to determine a drug-associated risk of major birth defects or miscarriage. However, there are risks to the mother and fetus associated with epinephrine use during labor or delivery and risks due to untreated hypotension associated with septic shock (see Clinical Considerations). In animal reproduction studies, epinephrine demonstrated adverse developmental effects when administered to pregnant rabbits (gastroschisis), mice (teratogenic effects, embryonic lethality, and delayed skeletal ossification), and hamsters (embryonic lethality and delayed skeletal ossification) during organogenesis at doses approximately 15 times, 3 times and 2 times, respectively, the maximum recommended daily dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypotension associated with septic shock is a medical emergency in pregnancy which can be fatal if left untreated. Delaying treatment in pregnant women with hypotension associated with septic shock may increase the risk of maternal and fetal morbidity and mortality. Do not withhold life-sustaining therapy for a pregnant woman.
Labor or Delivery
Epinephrine usually inhibits spontaneous, or oxytocin induced contractions of the pregnant human uterus and may delay the second stage of labor. Avoid epinephrine during the second stage of labor. In dosage sufficient to reduce uterine contractions, the drug may cause a prolonged period of uterine atony with hemorrhage. Avoid epinephrine in obstetrics when maternal blood pressure exceeds 130/80 mmHg.
Although epinephrine may improve maternal hypotension associated with septic shock, it may result in uterine vasoconstriction, decreased uterine blood flow, and fetal anoxia.
Data
Animal Data
In an embryofetal development study with pregnant rabbits dosed during the period of organogenesis (on days 3 to 5, 6 to 7 or 7 to 9 of gestation), epinephrine caused teratogenic effects (including gastroschisis) at doses approximately 15 times the maximum recommended intramuscular, subcutaneous, or intravenous dose (on a mg/m2 basis at a maternal subcutaneous dose of 1.2 mg/kg/day for two to three days). Animals treated on days 6 to 7 had decreased number of implantations.
In an embryofetal development study, pregnant mice were administered epinephrine (0.1 to 10 mg/kg/day) on Gestation Days 6 to 15. Teratogenic effects, embryonic lethality, and delays in skeletal ossification were observed at approximately 3 times the maximum recommended intramuscular, subcutaneous, or intravenous dose (on a mg/m2 basis at maternal subcutaneous dose of 1 mg/kg/day for 10 days). These effects were not seen in mice at approximately 2 times the maximum recommended daily intramuscular or subcutaneous dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
In an embryofetal development study with pregnant hamsters dosed during the period of organogenesis from gestation days 7 to 10, epinephrine produced reductions in litter size and delayed skeletal ossification at doses approximately 2 times the maximum recommended intramuscular, subcutaneous, or intravenous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day).
8.2. Lactation
Risk Summary
There is no information regarding the presence of epinephrine in human milk or the effects of epinephrine on the breastfed infant or on milk production. However, due to its poor oral bioavailability and short half-life, epinephrine exposure is expected to be very low in the breastfed infant. The lack of clinical data during lactation precludes a clear determination of the risk of epinephrine to a breastfed infant.
8.4. Pediatric Use
Safety and effectiveness of epinephrine in pediatric patients with septic shock have not been established.
8.5. Geriatric Use
Clinical studies of epinephrine for the treatment of hypotension associated with septic shock did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10. OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Epinephrine overdosage can also cause transient bradycardia followed by tachycardia and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Myocardial ischemia and infarction, cardiomyopathy, extreme pallor and coldness of the skin, metabolic acidosis due to elevated blood lactic acid levels, and renal insufficiency and failure have also been reported.
Epinephrine is rapidly inactivated in the body and treatment following overdose with epinephrine is primarily supportive. Treatment of pulmonary edema consists of a rapidly acting alpha- adrenergic blocking drug (such as phentolamine mesylate) and respiratory support. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug (such as propranolol). If necessary, pressor effects may be counteracted by rapidly acting vasodilators (such as nitrates) or α-adrenergic blocking drugs. If prolonged hypotension follows such measures, it may be necessary to administer another pressor drug.
-
11. DESCRIPTION
Adrenalin (epinephrine in sodium chloride injection) is a sympathomimetic catecholamine. The chemical name of epinephrine is: 1,2- Benzenediol, 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]-, or (-)-3,4-Dihydroxy-α-[2- (methylamino)ethyl]benzyl alcohol.
The chemical structure of epinephrine is:
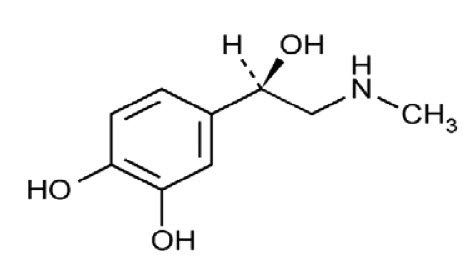
The molecular weight of epinephrine is 183.2.
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin.
Adrenalin (epinephrine in sodium chloride injection) is a clear, colorless, sterile solution administered by intravenous infusion, supplied in a 250 mL infusion bag. It is provided in five (5) ready-to-use concentrations containing:
Ingredient Concentration 8 mcg/mL 16 mcg/mL 20 mcg/mL 32 mcg/mL 40 mcg/mL Epinephrine, USP 8 mcg 16 mcg 20 mcg 32 mcg 40 mcg Sodium chloride, USP 9 mg 9 mg 9 mg 9 mg 9 mg Disodium Edetate Dihydrate (EDTA), USP 10 mcg 10 mcg 10 mcg 10 mcg 10 mcg L (+) Tartaric Acid, NF 6.6 mcg 13.1 mcg 16.4 mcg 26.2 mcg 32.8 mcg It may contain hydrochloric acid and/or sodium hydroxide for pH adjustment. It has a pH range of 3.7 - 4.3. The headspace in the containers has been displaced with nitrogen gas.
-
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Epinephrine acts on both alpha and beta-adrenergic receptors. The mechanism of the rise in blood pressure is 3-fold: a direct myocardial stimulation that increases the strength of ventricular contraction (positive inotropic action), an increased heart rate (positive chronotropic action), and peripheral vasoconstriction.
12.2. Pharmacodynamics
Intravenous use for hypotension associated with septic shock
Following intravenous administration of epinephrine, increases in systolic blood pressure and heart rate are observed. Decreases in systemic vascular resistance and diastolic blood pressure are observed at low doses of epinephrine because of β2-mediated vasodilation, but are overtaken by α1-mediated peripheral vasoconstriction at higher doses leading to increase in diastolic blood pressure. The onset of blood pressure increase following an intravenous dose of epinephrine is < 5 minutes and the time to offset blood pressure response occurs within 15 minutes. Most vascular beds are constricted including renal, splanchnic, mucosal and skin.
Epinephrine causes mydriasis when administered parenterally.
12.3. Pharmacokinetics
Following intravenous injection, epinephrine is rapidly cleared from the plasma with an effective half-life of < 5 minutes. A pharmacokinetic steady state following continuous intravenous infusion is achieved within 10-15 minutes. In patients with septic shock, epinephrine displays dose-proportional pharmacokinetics in the infusion dose range of 0.03 to 1.7 mcg/kg/min.
Epinephrine is extensively metabolized with only a small amount excreted unchanged.
Epinephrine is rapidly degraded to vanillylmandelic acid, an inactive metabolite, by monoamine oxidase and catechol-O-methyltransferase that are abundantly expressed in the liver, kidneys and other extraneuronal tissues. The tissues with the highest contribution to removal of circulating exogenous epinephrine are the liver (32%), kidneys (25%), skeletal muscle (20%), and mesenteric organs (12%).
Specific Populations
Elderly
In a pharmacokinetic study of 45-minute epinephrine infusions given to healthy men aged 20 to 25 years and healthy men aged 60 to 65 years, the mean plasma metabolic clearance rate of epinephrine at steady state was greater among the older men (144.8 versus 78 mL/kg/min for a 0.0143 mcg/kg/min infusion).
Body Weight
Body weight has been found to influence epinephrine pharmacokinetics. Higher body weight was associated with a higher plasma epinephrine clearance and a lower concentration plateau.
-
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro.
Epinephrine was positive in the Salmonella bacterial reverse mutation assay, positive in the mouse lymphoma assay, and negative in the in vivo micronucleus assay. Epinephrine is an oxidative mutagen based on the E. coli WP2 Mutoxitest bacterial reverse mutation assay. This should not prevent the use of epinephrine under the conditions noted under Indications and Usage (1).
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (15-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
Adrenalin (epinephrine in sodium chloride injection) is supplied as a clear, colorless sterile solution in a single-dose 250 mL non-PVC infusion bag with a single function connector system consisting of a port and cap, packaged individually in an aluminum overwrap with an oxygen scavenger.
Supplied as:
Unit of Sale Strength Pack Factor NDC 42023-273-10 2 mg/250 mL (8 mcg/mL) 10 units NDC 42023-315-10 4 mg/250 mL (16 mcg/mL) 10 units NDC 42023-434-10 5 mg/250 mL (20 mcg/mL) 10 units NDC 42023-500-10 8 mg/250 mL (32 mcg/mL) 10 units NDC 42023-721-10 10 mg/250 mL (40 mcg/mL) 10 units Store between 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature]. Epinephrine is light sensitive. Protect from light and freezing.
Keep in foil overwrap until ready to use. Discard after 24 hours of opening overwrap.
Manufactured for:
Endo USA
Malvern, PA 19355
© 2024 Endo, Inc. or one of its affiliates.
Revised: 04/2024
- PRINCIPAL DISPLAY PANEL - 4 mg per 250 mL (16 mcg/mL)
-
INGREDIENTS AND APPEARANCE
ADRENALIN (EPINEPHRINE IN SODIUM CHLORIDE)
epinephrine in sodium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42023-315 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 16 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL TARTARIC ACID (UNII: W4888I119H) 13.1 ug in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 10 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-315-10 10 in 1 CARTON 10/04/2024 1 NDC:42023-315-01 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215875 10/04/2024 Labeler - Endo USA, Inc. (119185057)


