Label: TEZSPIRE- tezepelumab-ekko injection, solution
- NDC Code(s): 55513-100-01, 55513-100-96, 55513-100-99, 55513-112-01, view more
- Packager: Amgen, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TEZSPIRE safely and effectively. See full prescribing information for TEZSPIRE. TEZSPIRE® (tezepelumab-ekko) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE TEZSPIRE is indicated for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma. Limitations of Use: TEZSPIRE is not indicated for the ...
-
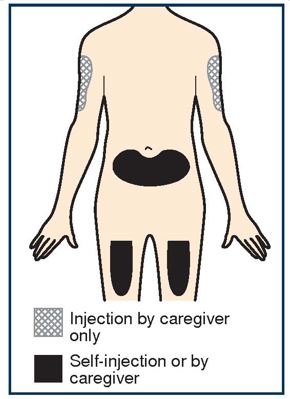
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - The recommended dosage of TEZSPIRE is 210 mg administered subcutaneously once every 4 weeks. Missed Dose Information - If a dose is missed, administer the dose as soon ...
-
3 DOSAGE FORMS AND STRENGTHS Injection: a clear to opalescent, colorless to light yellow solution available as: • 210 mg/1.91 mL (110 mg/mL) solution in a single-dose glass vial. • 210 mg/1.91 mL (110 mg/mL) solution in a ...
-
4 CONTRAINDICATIONS TEZSPIRE is contraindicated in patients who have known hypersensitivity to tezepelumab-ekko or any of its excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity Reactions - Hypersensitivity reactions were observed in the clinical trials (e.g., rash and allergic conjunctivitis) following the administration of TEZSPIRE. Postmarketing ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Hypersensitivity Reactions [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS No formal drug interaction studies have been performed with TEZSPIRE.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no available data on TEZSPIRE use in pregnant women to evaluate for any drug-associated risk of major birth defects, miscarriage, or other adverse ...
-
11 DESCRIPTION Tezepelumab-ekko, a thymic stromal lymphopoietin (TSLP) blocker, is a human monoclonal antibody immunoglobulin G2λ (IgG2λ) produced in Chinese hamster ovary (CHO) cells by recombinant DNA ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tezepelumab-ekko is a thymic stromal lymphopoietin (TSLP) blocker, human monoclonal antibody IgG2λ that binds to human TSLP with a dissociation constant of 15.8 pM and ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to evaluate the carcinogenic potential of tezepelumab-ekko. The malignancy risk in humans from ...
-
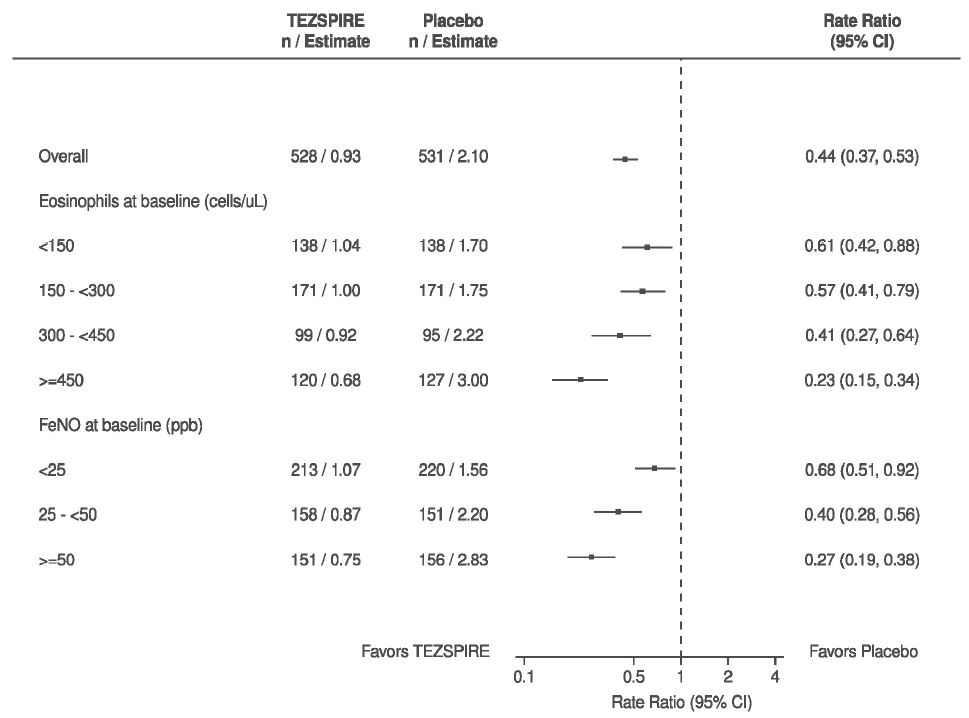
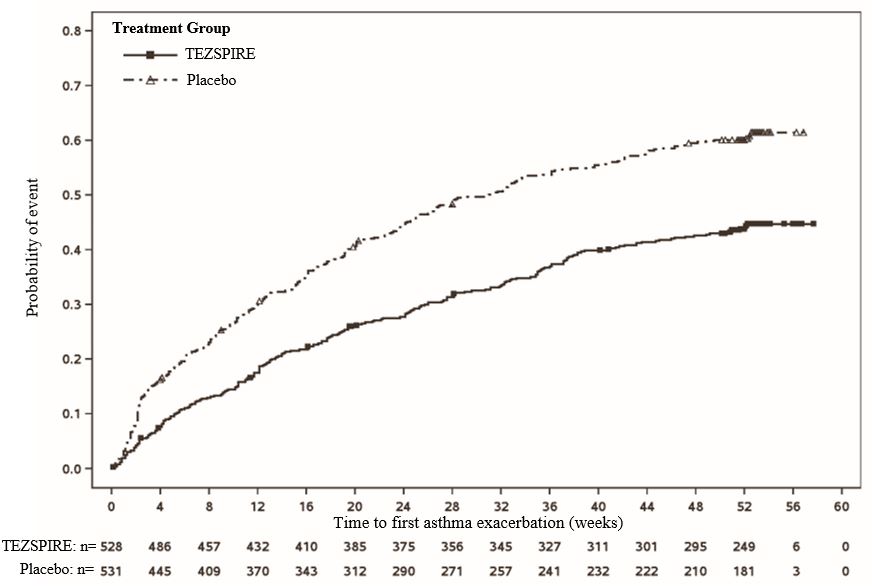
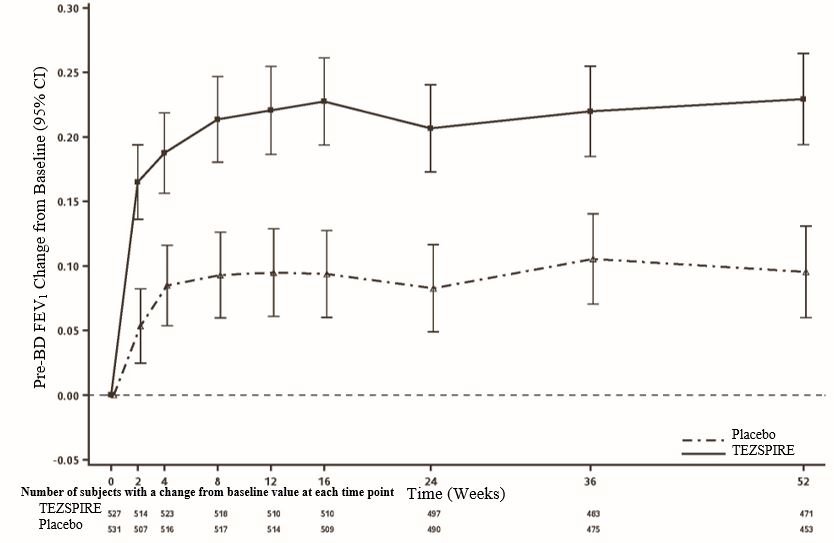
14 CLINICAL STUDIES The efficacy of TEZSPIRE was evaluated in two randomized, double-blind, parallel group, placebo-controlled clinical trials (PATHWAY [NCT02054130] and NAVIGATOR [NCT03347279]) of 52 weeks duration ...
-
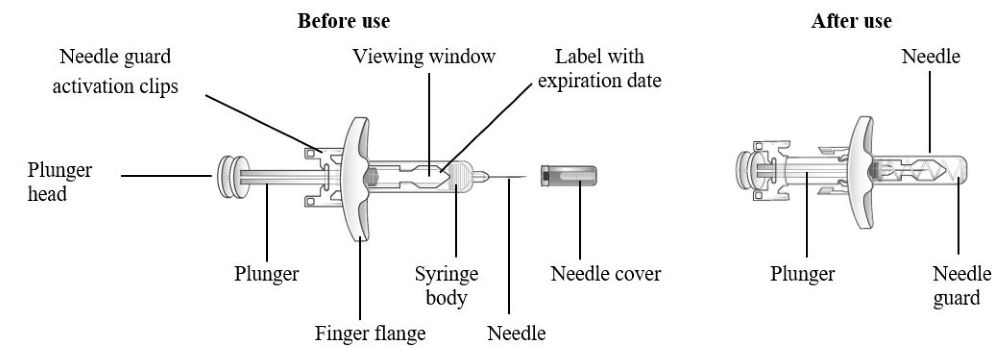
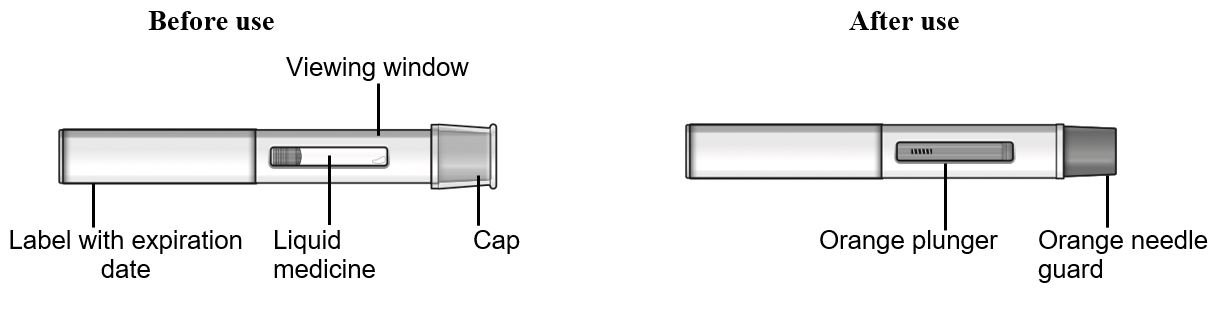
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - TEZSPIRE (tezepelumab-ekko) injection is a sterile, preservative-free, clear to opalescent, colorless to light yellow solution supplied as a single-dose vial, single-dose pre-filled ...
-
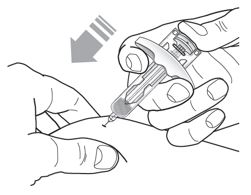
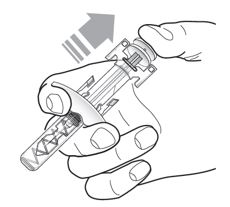
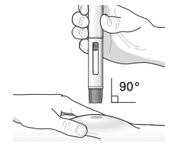
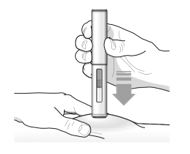
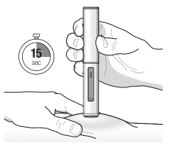
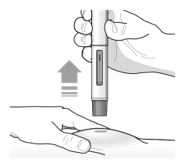
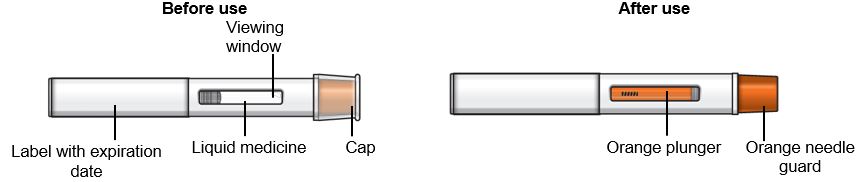
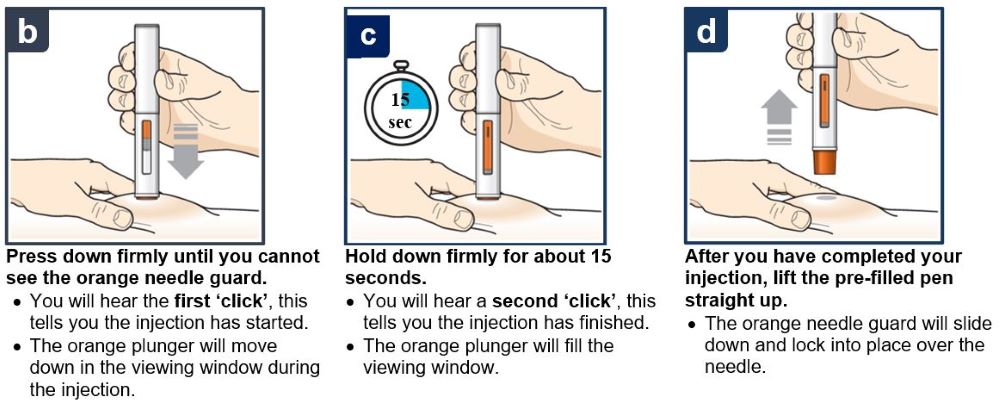
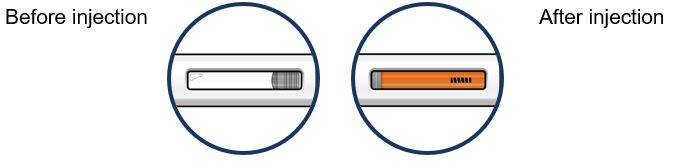
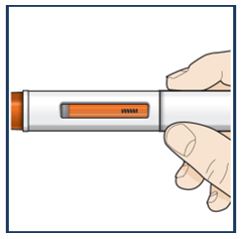
17 PATIENT COUNSELING INFORMATION Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Hypersensitivity Reactions - Inform patients that hypersensitivity ...
-
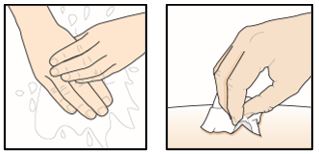
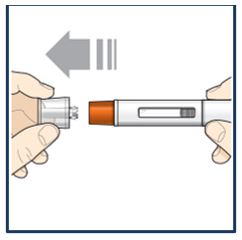
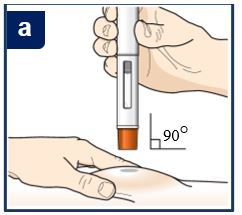
PATIENT PACKAGE INSERTPATIENT INFORMATION - TEZSPIRE® (TEZ-SPY-ER) (tezepelumab-ekko) injection, for subcutaneous use - What is TEZSPIRE? TEZSPIRE is a prescription medicine used with other asthma ...
-
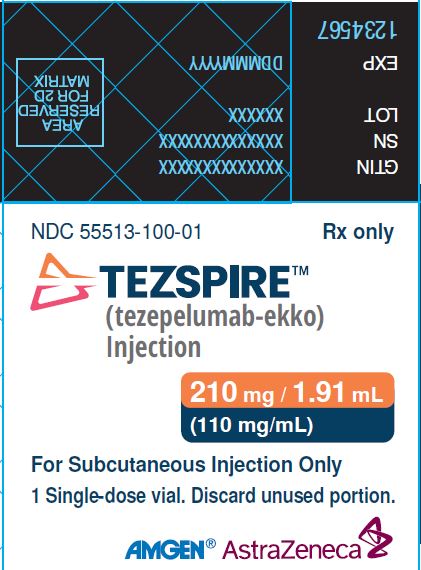
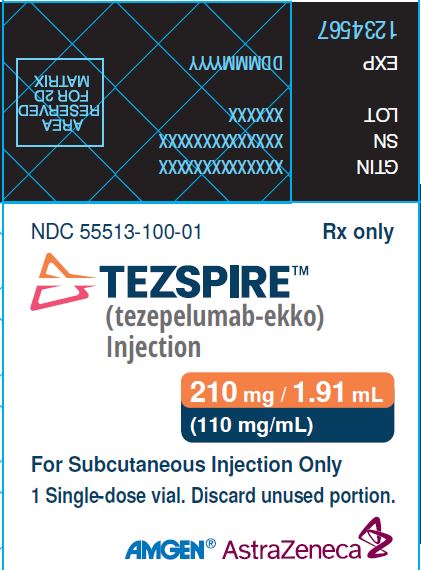
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 55513-100-01 Rx only - TEZSPIRETM - (tezepelumab-ekko) Injection - 210 mg/1.91 mL (110 mg/mL) For Subcutaneous Injection Only - 1 singe-dose vial. Discard unused ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 55513-112-01 - TEZSPIRETM (tezepelumab-ekko) Injection - 210 mg/1.91 mL (110 mg/mL) Rx Only - For Subcutaneous Injection Only - Store the pre-filled syringe refrigerated at 36° F to 46° F - (2° C to 8 ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 55513-123-01 Rx only - TEZSPIRE® (tezepelumab-ekko) Injection - 210 mg/1.91 mL (110 mg/mL) For Subcutaneous Injection Only - Store the pre-filled pen refrigerated at ...
-
INGREDIENTS AND APPEARANCEProduct Information