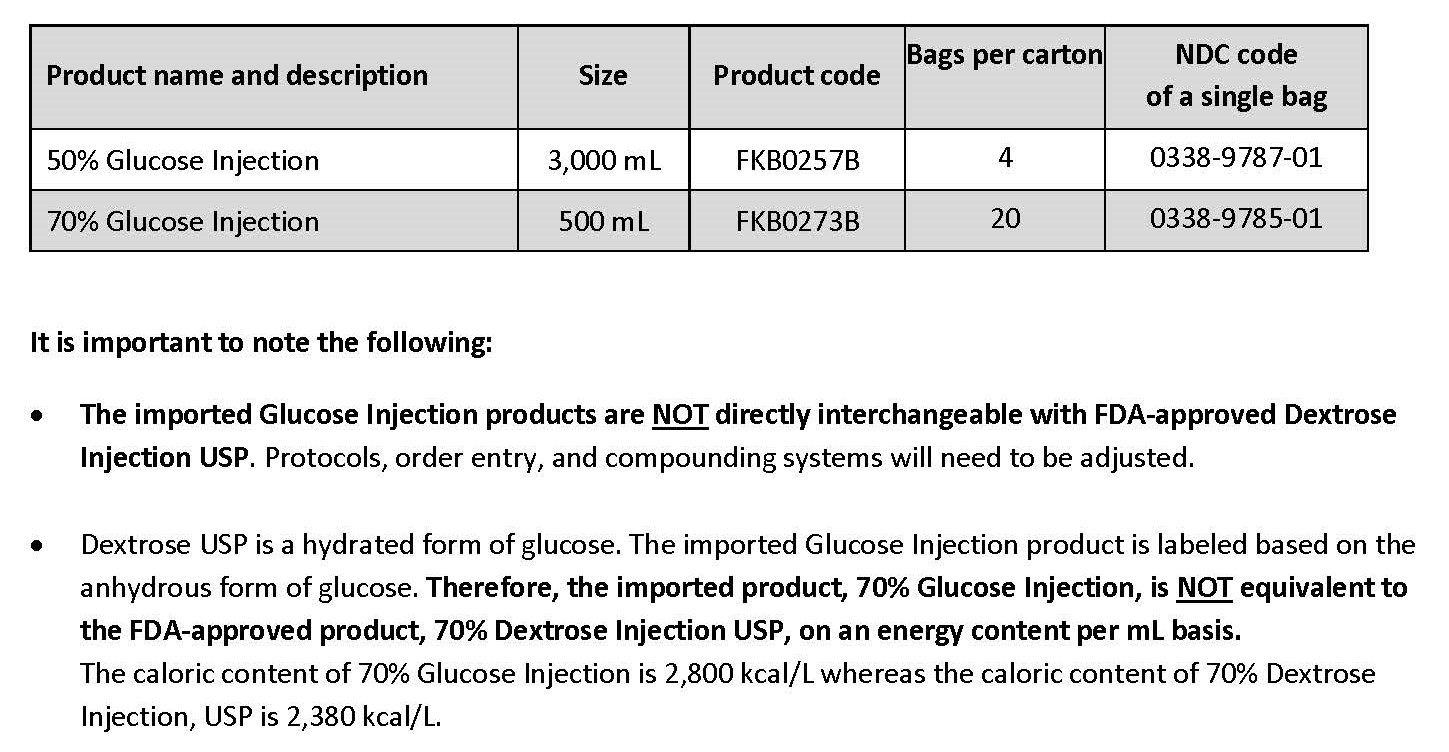
Label: DEXTROSE- dextrose monohydrate injection, solution
- NDC Code(s): 0338-9785-01, 0338-9785-20, 0338-9787-01, 0338-9787-04
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
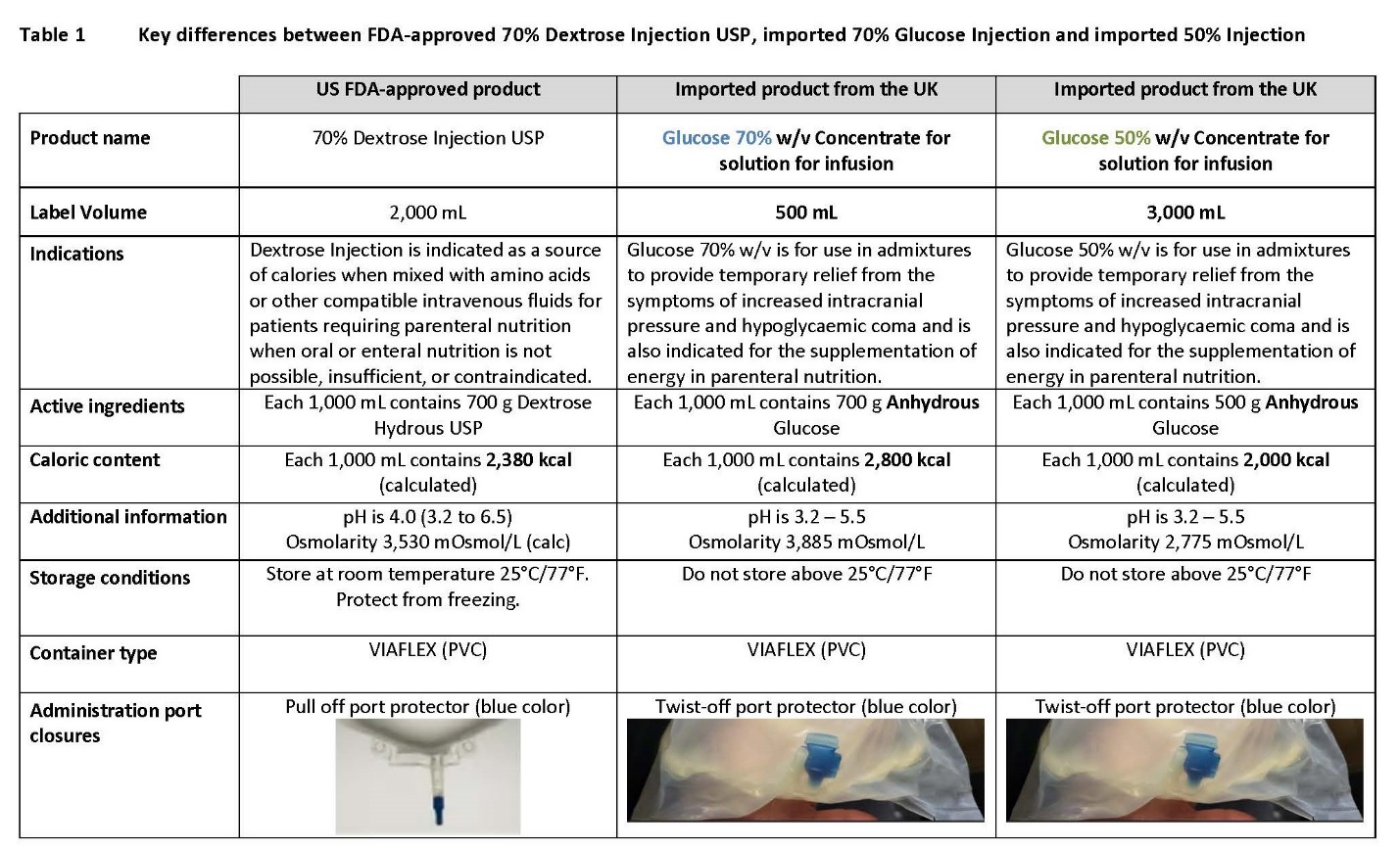
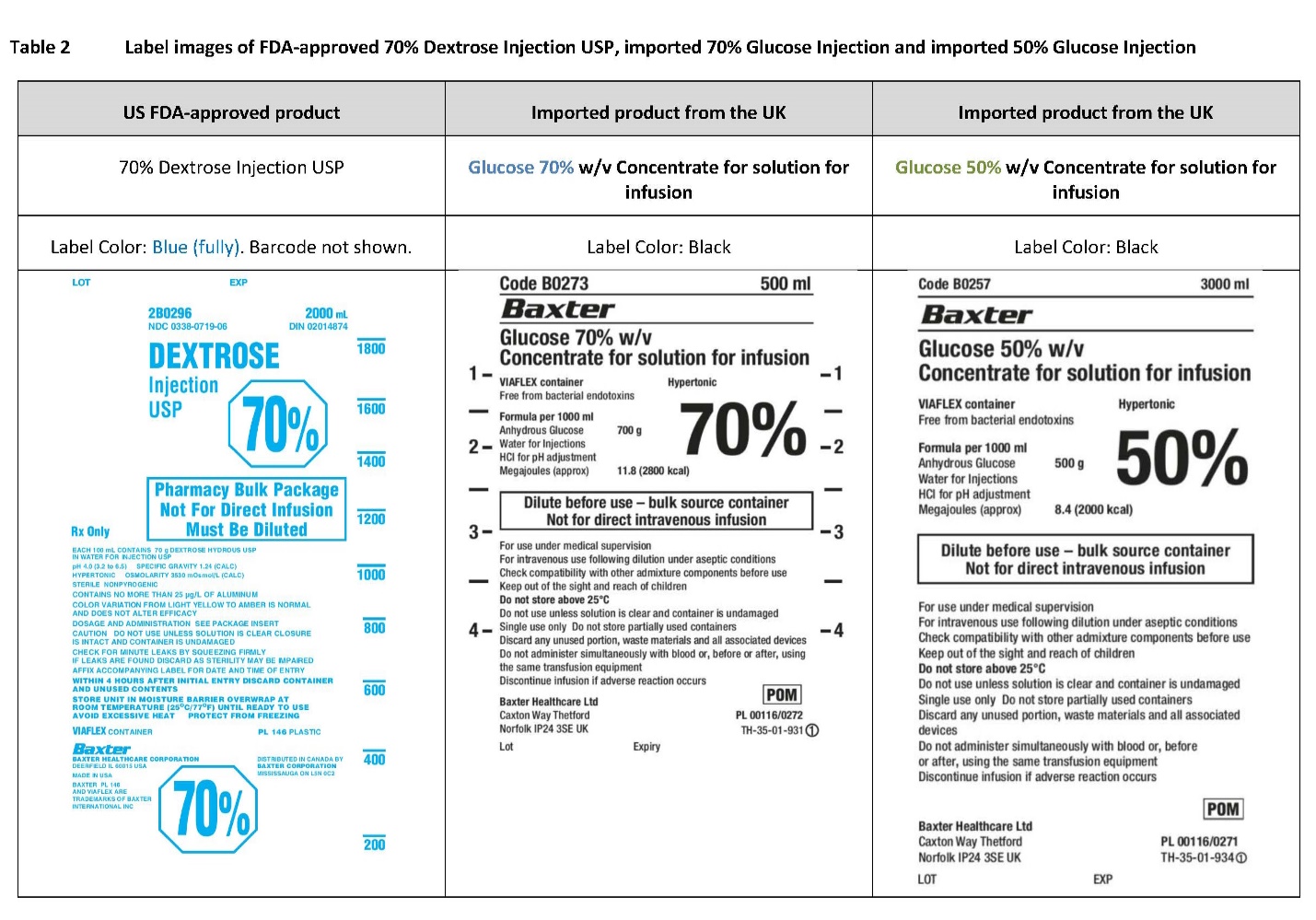
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
Code B0257 3000 ml
Baxter Logo
Glucose 50% w/v
Concentrate for solution for infusionVIAFLEX container Hypertonic
Free from bacterial endotoxinsFormula per 1000 ml
Anhydrous Glucose
Water for Injections
HCI for pH adjustment
Megajoules (approx.)500 g
8.4 (2000 kcal)
50%
Dilute before use – bulk source container
Not for direct intravenous infusionFor use under medical supervision
For intravenous use following dilution under aseptic conditions
Check compatibility with other admixture components before use
Keep out of the sight and reach of children
Do not store above 25°C
Do not use unless solution is clear and container is undamaged
Single use only Do not store partially used containers
Discard any unused portion, waste materials and all associated
devices
Do not administer simultaneously with blood or, before
or after, using the same transfusion equipment
Discontinue infusion if adverse reaction occursBaxter Healthcare Ltd
Caxton Way Thetford
Norfolk IP24 3SE UKLOT EXP
POM
PL 00116/0271
TH-35-01-934Carton Label
Baxter Logo
Code FKB0257BGlucose 50% w/v
Concentrate for
solution for infusionHypertonic
Do not store above 25°C
Keep out of sight and reach of childrenBaxter Healthcare Ltd
Caxton Way, Thetford, Norfolk, IP24 3SE, UKPOM
LOT: EXP:
4 x 3000 ml
(≈15.5 kg)50%
PL 00116/0271
TH-25-02-116Baxter Logo
Code FKB0257BGlucose 50% w/v
Concentrate for
solution for infusionHypertonic
POM
4 x 3000 ml
(≈15.5 kg)50%
LOT: EXP:
Container Label
1 –
-
2-
-
3-
-
4-
Code B0273 500 ml
Baxter Logo
Glucose 70% w/v
Concentrate for solution for infusionVIAFLEX container Hypertonic
Free from bacterial endotoxinsFormula per 1000 ml
Anhydrous Glucose
Water for Injections
HCI for pH adjustment
Megajoules (approx.)700 g
11.8 (2800 kcal)
70%
Dilute before use – bulk source container
Not for direct intravenous infusionFor use under medical supervision
For intravenous use following dilution under aseptic conditions
Check compatibility with other admixture components before use
Keep out of the sight and reach of children
Do not store above 25°C
Do not use unless solution is clear and container is undamaged
Single use only Do not store partially used containers
Discard any unused portion, waste materials and all associated devices
Do not administer simultaneously with blood or, before or after, using
the same transfusion equipment
Discontinue infusion if adverse reaction occursBaxter Healthcare Ltd
Caxton Way Thetford
Norfolk IP24 3SE UKLot Expiry
POM
PL 00116/0272
TH-35-01-931-1
-
-2
-
-3
-
-4
Carton Label
Baxter Logo
Code FKB0273BGlucose 70% w/v
Concentrate for
solution for infusionHypertonic
Do not store above 25°C
Keep out of sight and reach of childrenBaxter Healthcare Ltd
Caxton Way, Thetford, Norfolk, IP24 3SE, UKLOT: EXP:
20 x 500 ml
(≈15.0 kg)70%
POM
PL 00116/0272
TH-25-02-117Baxter Logo
Code FKB0273BGlucose 70% w/v
Concentrate for
solution for infusionHypertonic
20 x 500 ml
(≈15.0 kg)70%
POM
PL 00116/0272LOT: EXP:
-
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9787 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 50 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9787-04 4 in 1 CARTON 10/18/2024 1 NDC:0338-9787-01 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/18/2024 DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9785 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 70 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9785-20 20 in 1 CARTON 10/18/2024 1 NDC:0338-9785-01 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/18/2024 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Ltd 221478644 ANALYSIS(0338-9787, 0338-9785) , LABEL(0338-9787, 0338-9785) , MANUFACTURE(0338-9787, 0338-9785) , PACK(0338-9787, 0338-9785) , STERILIZE(0338-9787, 0338-9785)