Label: SODIUM BICARBONATE injection, solution
- NDC Code(s): 70095-050-01, 70095-050-03
- Packager: Sun Pharmaceutical Industries Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONSodium Bicarbonate Injection, USP is a sterile, nonpyrogenic, hypertonic solution of sodium bicarbonate (NaHCO3) in water for injection for administration by the intravenous route as an ...
-
CLINICAL PHARMACOLOGYIntravenous sodium bicarbonate therapy increases plasma bicarbonate, buffers excess hydrogen ion concentration, raises blood pH and reverses the clinical manifestations of acidosis. Sodium ...
-
INDICATIONS AND USAGESodium Bicarbonate Injection is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe ...
-
CONTRAINDICATIONSSodium Bicarbonate Injection is contraindicated in patients who are losing chloride by vomiting or from continuous gastrointestinal suction, and in patients receiving diuretics known ...
-
WARNINGSSolutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states ...
-
PRECAUTIONSGeneral - Do not use unless solution is clear and the container or seal is intact. Discard unused portion. The potentially large loads of sodium given with bicarbonate ...
-
ADVERSE REACTIONSOverly aggressive therapy with Sodium Bicarbonate Injection, can result in metabolic alkalosis (associated with muscular twitchings, irritability and tetany) and hypernatremia. Inadvertent ...
-
OVERDOSAGEShould alkalosis result, the bicarbonate should be stopped and the patient managed according to the degree of alkalosis present. 0.9% sodium chloride injection intravenous may be given; potassium ...
-
DOSAGE AND ADMINISTRATIONSodium Bicarbonate Injection is administered by the intravenous route. In cardiac arrest, a rapid intravenous dose of one to two 50 mL vials (44.6 to 100 mEq) may be given initially and continued ...
-
HOW SUPPLIEDSodium Bicarbonate Injection, USP is supplied in the following dosage forms: NDC Code: Conc % mg/mL - (NaHCO3) mEq/mL - (Na+) mEq/mL ...
-
PRINCIPAL DISPLAY PANEL - Vial LabelNDC 70095-050-01 - 8.4% Sodium Bicarbonate Injection, USP - 50 mEq/50 mL (1 mEq/mL) For Intravenous Use Only. Rx only - 50 mL Single-Dose Vial - steriscience - SUN PHARMA
-
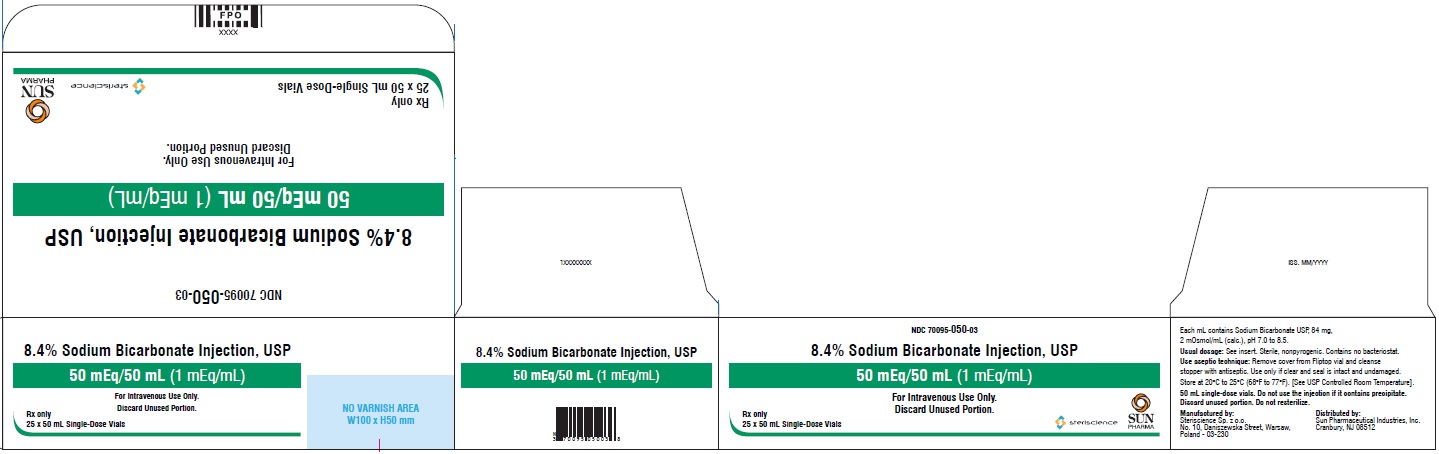
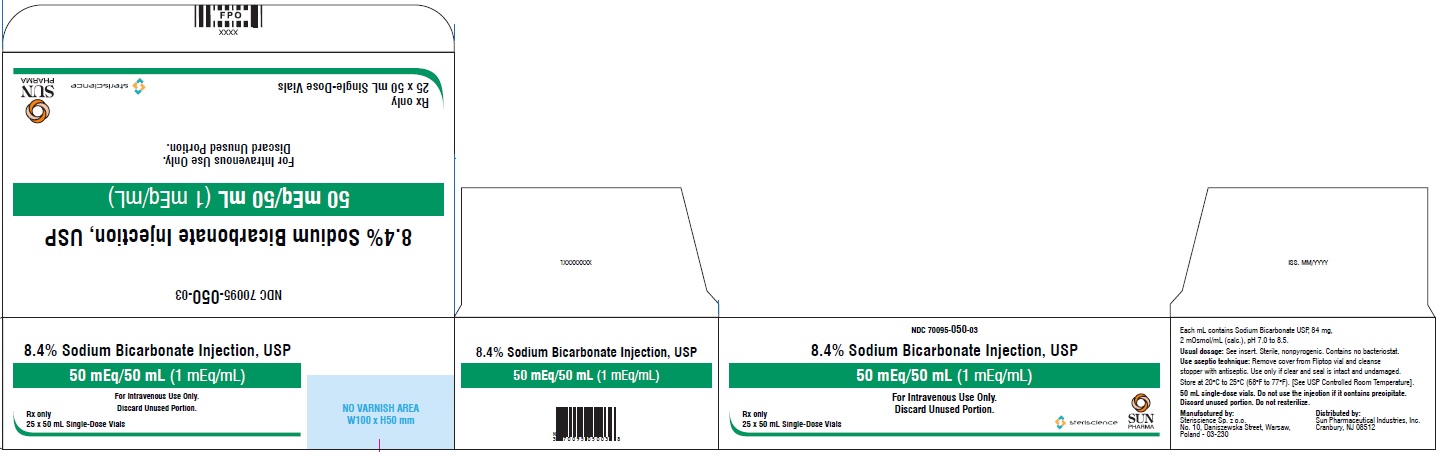
PRINCIPAL DISPLAY PANEL - CartonNDC 70095-050-03 - 8.4% Sodium Bicarbonate Injection, USP - 50 mEq/50 mL (1 mEq/mL) for Intravenous Use Only. Discard Unused Portion. Rx Only - 25 × 50 mL Single-Dose Vials - steriscience ...
-
INGREDIENTS AND APPEARANCEProduct Information