Label: THIAMINE HYDROCHLORIDE injection, solution
- NDC Code(s): 25021-500-02
- Packager: Sagent Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Thiamine Hydrochloride Injection, USP is a sterile solution of thiamine hydrochloride in Water for Injection for intramuscular (IM) or slow intravenous (IV) administration.
Each mL contains: Thiamine hydrochloride 100 mg; chlorobutanol anhydrous (chloral derivative) 0.5%; monothioglycerol 0.5%; Water for Injection q.s. Sodium hydroxide may have been added for pH adjustment (2.5 to 4.5).
Thiamine hydrochloride, or vitamin B1, occurs as white crystals or crystalline powder that usually has a slight characteristic odor.
Freely soluble in water; soluble in glycerin; slightly soluble in alcohol; insoluble in ether and benzene. Thiamine is rapidly destroyed in neutral or alkaline solutions but is stable in the dry state. It is reasonably stable to heat in acid solution.
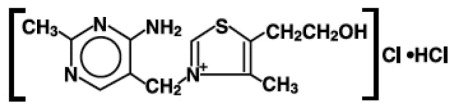
The chemical name of thiamine hydrochloride is thiazolium,3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4- methylchloride, monohydrochloride and it has the following structural formula:
-
CLINICAL PHARMACOLOGY
The water soluble vitamins are widely distributed in both plants and animals. They are absorbed in man by both diffusion and active transport mechanisms. These vitamins are structurally diverse (derivatives of sugar, pyridine, purines, pyrimidine, organic acid complexes and nucleotide complex) and act as coenzymes, as oxidation-reduction agents, possibly as mitochondrial agents. Metabolism is rapid, and the excess is excreted in the urine.
Thiamine is distributed in all tissues. The highest concentrations occur in liver, brain, kidney and heart. When thiamine intake is greatly in excess of need, tissue stores increase two to three times. If intake is insufficient, tissues become depleted of their vitamin content. Absorption of thiamine following IM administration is rapid and complete.
Thiamine combines with adenosine triphosphate (ATP) to form thiamine pyrophosphate, also known as cocarboxylase, a coenzyme. Its role in carbohydrate metabolism is the decarboxylation of pyruvic acid in the blood and α-ketoacids to acetaldehyde and carbon dioxide. Increased levels of pyruvic acid in the blood indicate vitamin B1 deficiency.
The requirement for thiamine is greater when the carbohydrate content of the diet is raised. Body depletion of vitamin B1 can occur after approximately three weeks of total absence of thiamine in the diet.
-
INDICATIONS AND USAGE
Thiamine Hydrochloride Injection is effective for the treatment of thiamine deficiency or beriberi whether of the dry (major symptoms related to the nervous system) or wet (major symptoms related to the cardiovascular system) variety. Thiamine Hydrochloride Injection should be used where rapid restoration of thiamine is necessary, as in Wernicke's encephalopathy, infantile beriberi with acute collapse, cardiovascular disease due to thiamine deficiency, or neuritis of pregnancy if vomiting is severe. It is also indicated when giving IV dextrose to individuals with marginal thiamine status to avoid precipitation of heart failure.
Thiamine Hydrochloride Injection is also indicated in patients with established thiamine deficiency who cannot take thiamine orally due to coexisting severe anorexia, nausea, vomiting, or malabsorption. Thiamine Hydrochloride Injection is not usually indicated for conditions of decreased oral intake or decreased gastrointestinal absorption, because multiple vitamins should usually be given.
-
CONTRAINDICATIONS
A history of sensitivity to thiamine or to any of the ingredients in this drug is a contraindication. (See WARNINGS for further information.)
-
WARNINGS
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Serious hypersensitivity/anaphylactic reactions can occur, especially after repeated administration. Deaths have resulted from IV or IM administration of thiamine (see ADVERSE REACTIONS).
Routine testing for hypersensitivity, in many cases, may not detect hypersensitivity. Nevertheless, a skin test should be performed on patients who are suspected of drug allergies or previous reactions to thiamine, and any positive responders should not receive thiamine by injection.
If hypersensitivity to thiamine is suspected (based on history of drug allergy or occurrence of adverse reactions after thiamine administration), administer one-hundredth of the dose intradermally and observe for 30 minutes. If no reaction occurs, full dose can be given; the patient should be observed for at least 30 minutes after injection. Be prepared to treat anaphylactic reactions regardless of the precautions taken.
Treatment of anaphylactic reactions includes maintaining a patent airway and the use of epinephrine, oxygen, vasopressors, steroids and antihistamines.
-
PRECAUTIONS
General
Simple vitamin B1 deficiency is rare. Multiple vitamin deficiencies should be suspected in any case of dietary inadequacy.
Information for Patients
The patient should be advised as to proper dietary habits during treatment so that relapses will be less likely to occur with reduction in dosage or cessation of injection therapy.
Usage in Pregnancy
Pregnancy Category A–Studies in pregnant women have not shown that thiamine hydrochloride increases the risk of fetal abnormalities if administered during pregnancy. If the drug is used during pregnancy, the possibility of fetal harm appears remote. Because studies cannot rule out the possibility of harm however, thiamine hydrochloride should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
An occasional individual may develop a hypersensitivity or life-threatening anaphylactic reaction to thiamine, especially after repeated injection. Collapse and death have been reported. A feeling of warmth, pruritus, urticaria, weakness, sweating, nausea, restlessness, tightness of the throat, angioneurotic edema, cyanosis, pulmonary edema, and hemorrhage into the gastrointestinal tract have also been reported. Some tenderness and induration may follow IM use (see WARNINGS).
To report SUSPECTED ADVERSE REACTIONS, contact Sagent Pharmaceuticals, Inc. at 1-866-625-1618 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Parenteral doses of 100 to 500 mg singly have been administered without toxic effects. However, dosages exceeding 30 mg three times a day are not utilized effectively.
When the body tissues are saturated with thiamine, it is excreted in the urine as pyrimidine. As the intake of thiamine is further increased, it appears unchanged in the urine.
-
DOSAGE AND ADMINISTRATION
“Wet” beriberi with myocardial failure must be treated as an emergency cardiac condition, and thiamine must be administered slowly by the IV route in this situation (see WARNINGS).
In the treatment of beriberi, 10 to 20 mg of thiamine hydrochloride injection are given IM three times daily for as long as two weeks. (See WARNINGS regarding repeated injection of thiamine.) An oral therapeutic multivitamin preparation containing 5 to 10 mg thiamine, administered daily for one month, is recommended to achieve body tissue saturation.
Infantile beriberi that is mild may respond to oral therapy, but if collapse occurs, doses of 25 mg may cautiously be given IV.
Poor dietary habits should be corrected and an abundant and well-balanced dietary intake should be prescribed.
Patients with neuritis of pregnancy in whom vomiting is severe enough to preclude adequate oral therapy should receive 5 to 10 mg of thiamine hydrochloride injection IM daily.
In the treatment of Wernicke-Korsakoff syndrome, thiamine hydrochloride injection has been administered IV in an initial dose of 100 mg, followed by IM doses of 50 to 100 mg daily until the patient is consuming a regular, balanced diet. (See WARNINGS regarding repeated injections of thiamine.)
Patients with marginal thiamine status to whom dextrose is being administered should receive 100 mg thiamine hydrochloride injection in each of the first few liters of IV fluid to avoid precipitating heart failure.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Thiamine Hydrochloride Injection, USP is supplied as follows:
Thiamine Hydrochloride Injection, USP NDC (100 mg per mL) Package Factor 25021-500-02 200 mg per 2 mL Multi-Dose Vial 25 vials per carton Storage Conditions
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light. Retain in carton until time of use.
Use only if solution is clear and seal intact.
Sterile, Nonpyrogenic.
The container closure is not made with natural rubber latex.SAGENT®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60195 (USA)
Made in India
©2020 Sagent Pharmaceuticals, Inc.Revised: January 2020
SAGENT Pharmaceuticals®
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THIAMINE HYDROCHLORIDE
thiamine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25021-500 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength thiamine hydrochloride (UNII: M572600E5P) (thiamine ion - UNII:4ABT0J945J) thiamine hydrochloride 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength chlorobutanol (UNII: HM4YQM8WRC) monothioglycerol (UNII: AAO1P0WSXJ) water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25021-500-02 25 in 1 CARTON 01/20/2021 1 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206106 01/20/2021 Labeler - Sagent Pharmaceuticals (080579617)