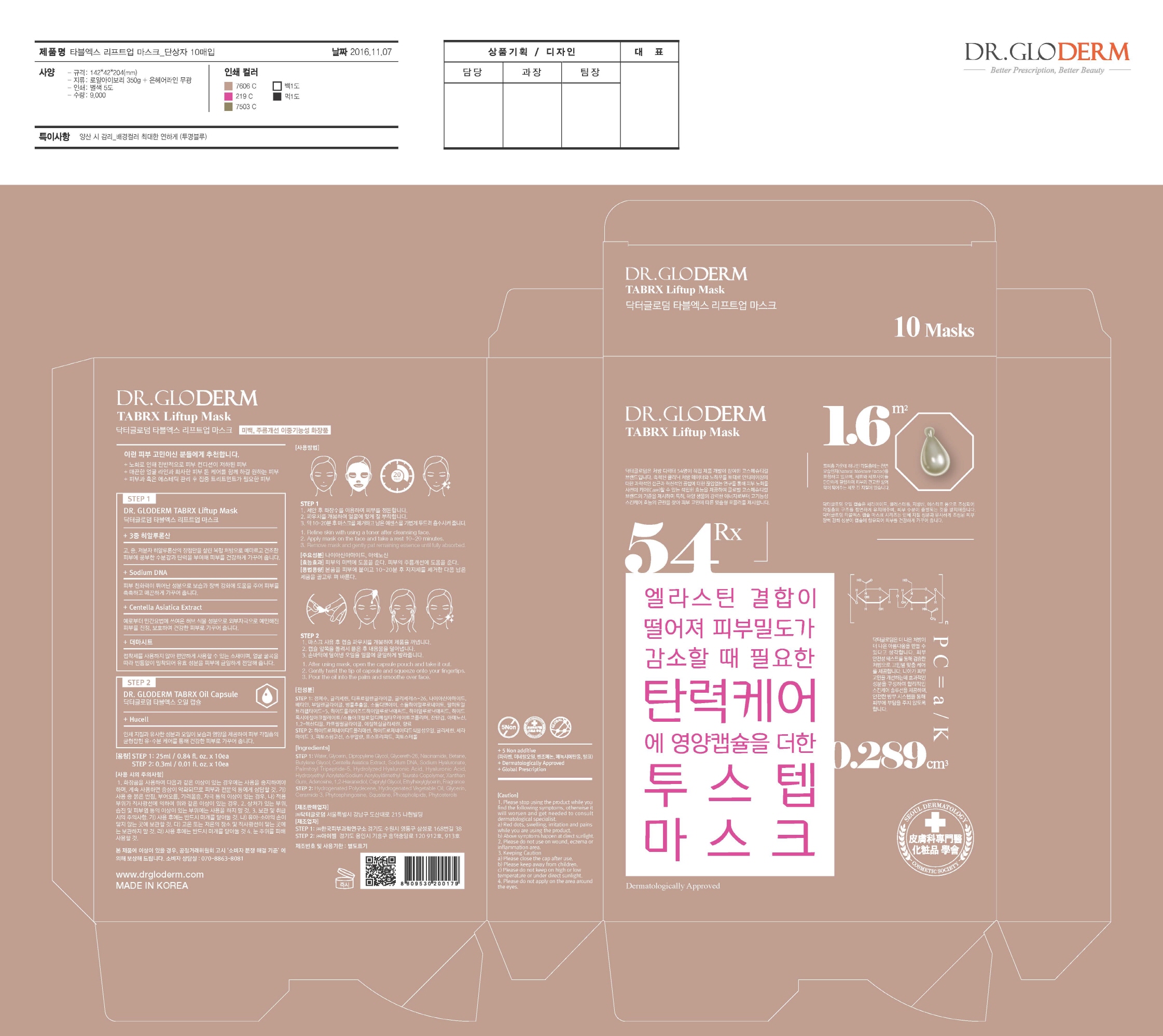
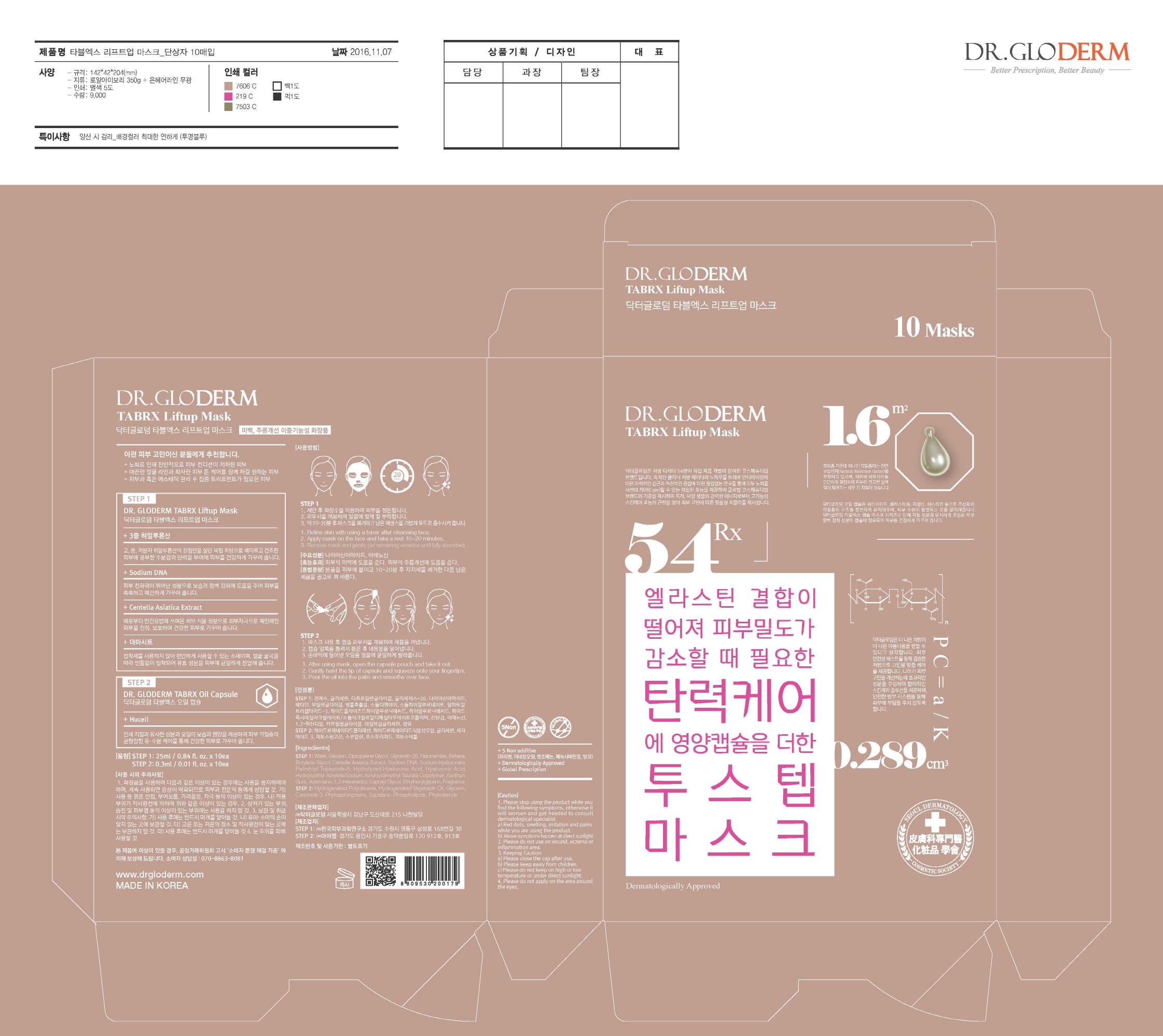
Label: DR. GLODERM TABRX LIFTUP MASK- niacinamide, adenosine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 71342-0006-1 - Packager: DR.GLODERM
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 27, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
1. If the following symptoms occur after product use, stop using the product immediately and consult a dermatologist (continuous use can exacerbate the symptoms).
1) Occurrence of red spots, swelling, itchiness, and other skin irritation
2) If the symptoms above occur after the application area is exposed to direct sunlight2. Do not use on open wounds, eczema, and other skin irritations
3. Precaution for Storage and Handling
1) Close the lid after use
2) Keep out of reach of infants and children
3) Do not to store in a place with high/low temperature and exposed to direct sunlight4. Use as avoiding eye areas.
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Step1
- Refine skin with using a toner after cleansing face.
- Apply mask on the face and take a rest 10~20 minutes.
- Remove mask and gently pat remaining essence until fully absorbed.
Step2
- After using mask, open the capsule pouch and take it out.
- Gently twist the tip of capsule and squeeze onto your fingertips.
- Pour the oil into the palm and smoothe over face.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR. GLODERM TABRX LIFTUP MASK
niacinamide, adenosine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71342-0006 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71342-0006-1 10 in 1 PACKAGE 03/27/2017 1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 POUCH 25 mL Part 2 1 POUCH 0.3 mL Part 1 of 2 DR. GLODERM TABRX LIFTUP MASK
niacinamide, adenosine liquidProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 25 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other Part 2 of 2 DR. GLODERM TABRX OILCAPSULE
glycerin oilProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 1 g in 100 mL Inactive Ingredients Ingredient Name Strength SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/27/2017 Labeler - DR.GLODERM (694773267) Registrant - DR.GLODERM (694773267) Establishment Name Address ID/FEI Business Operations EYESEL Co., Ltd. 557836898 manufacture(71342-0006) Establishment Name Address ID/FEI Business Operations KOREA DERMAL RESEARCH CENTER CO., LTD. 694640098 manufacture(71342-0006) Establishment Name Address ID/FEI Business Operations DR.GLODERM 694773267 label(71342-0006)