Label: HYDROCODONE BITARTRATE AND ACETAMINOPHEN tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 42254-346-08, 42254-346-20, 42254-346-30, 42254-346-60, view more - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 53746-119
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
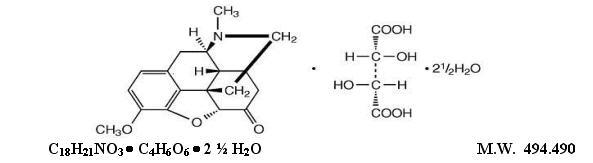
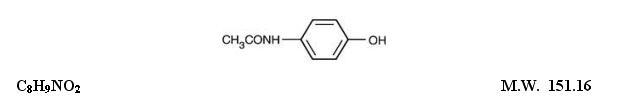
Hydrocodone Bitartrate and Acetaminophen Tablets, USP are supplied in tablet form for oral administration. WARNING: May be habit-forming(see PRECAUTIONS, Information For Patients, and DRUG ABUSE ...
-
CLINICAL PHARMACOLOGY
Hydrocodone is a semisynthetic narcotic analgesic and antitussive with multiple actions qualitatively similar to those of codeine. Most of these involve the central nervous system and smooth ...
-
INDICATIONS AND USAGE
Hydrocodone bitartrate and acetaminophen tablets, USP are indicated for the relief of moderate to moderately severe pain.
-
CONTRAINDICATIONS
This product should not be administered to patients who have previously exhibited hypersensitivity to hydrocodone or acetaminophen, or any other component of this product. Patients known to be ...
-
WARNINGS
Hepatotoxicity - Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the ...
-
PRECAUTIONS
General - Special Risk Patients: As with any narcotic analgesic agent, hydrocodone bitartrate and acetaminophen tablets should be used with caution in elderly or debilitated patients and those ...
-
ADVERSE REACTIONS
The most frequently reported adverse reactions are light-headedness, dizziness, sedation, nausea and vomiting. These effects seem to be more prominent in ambulatory than in non-ambulatory ...
-
DRUG ABUSE AND DEPENDENCE
Misuse, Abuse, and Diversion of Opioids - Hydrocodone bitartrate and acetaminophen tablets contain hydrocodone, an opioid agonist, and is a Schedule III controlled substance. Hydrocodone ...
-
OVERDOSAGE
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen. Signs and Symptoms - Hydrocodone: Serious overdose with hydrocodone is characterized by respiratory ...
-
DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to the severity of the pain and the response of the patient. However, it should be kept in mind that tolerance to hydrocodone can develop with continued use and ...
-
HOW SUPPLIED
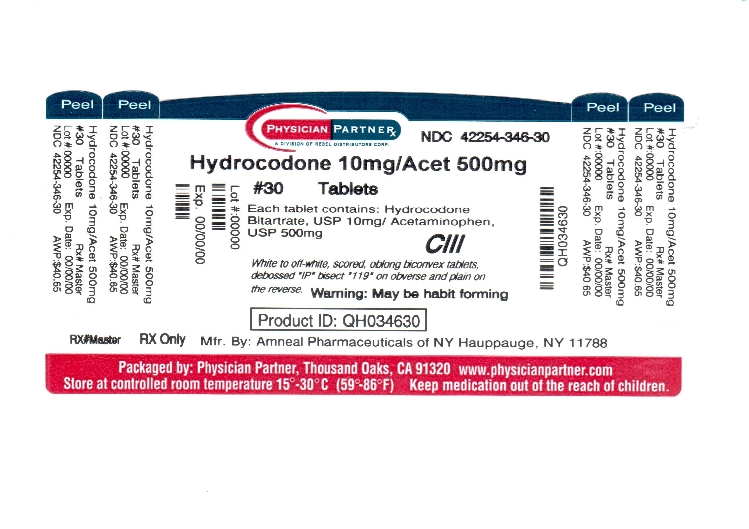
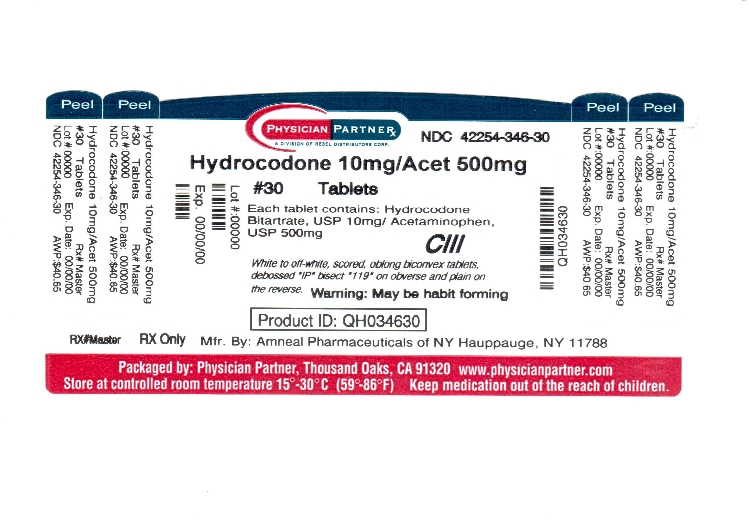
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg / 500 mg are supplied as white to off-white, scored, oblong biconvex tablets, debossed “IP” bisect “119” on obverse and plain on the ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Amneal Pharmaceuticals of NY - Hauppauge, NY 11788 - Rev. 05-2011 - Repackaged by: Rebel Distributors Corp. Thousand Oaks, CA 91320
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information