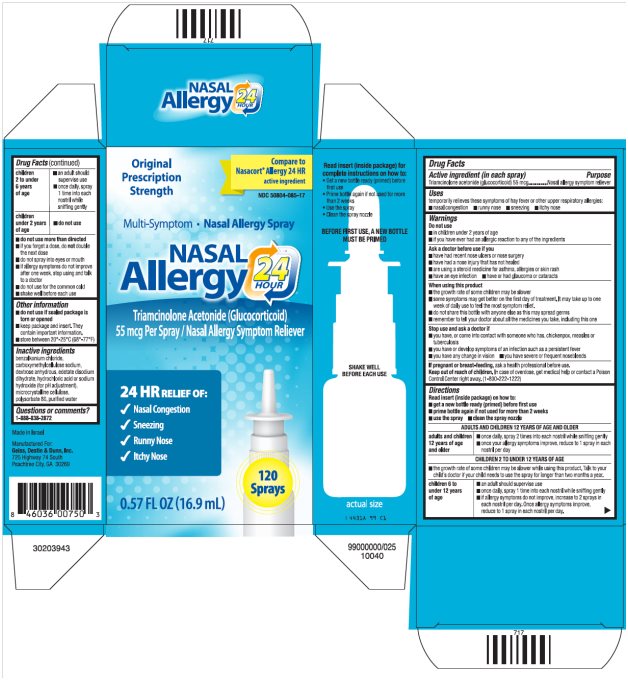
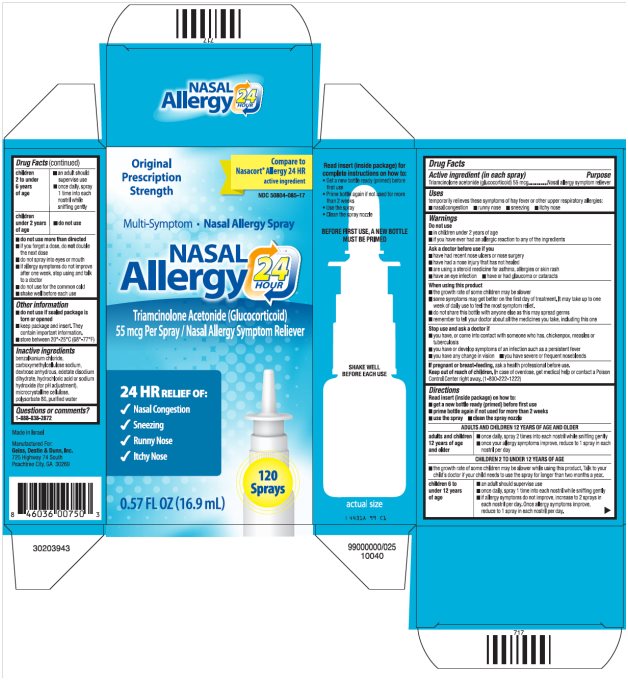
Label: NASAL ALLERGY 24 HOUR- triamcinolone acetonide spray, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 50804-085-17 - Packager: Geiss, Destin & Dunn Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 19, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each spray)
Triamcinolone acetonide (glucocorticoid) 55 mcg
-
Purpose
Nasal allergy symptom reliever
-
Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
-
Uses
temporarily relieves these symptoms of hay fever or other upper respiratory allergies: ■ nasal congestion - ■ runny nose - ■ sneezing - ■ itchy nose
-
Warnings
Do not use - ■ in children under 2 years of age - ■ if you have ever had an allergic reaction to any of the ingredients - Ask a doctor before use if you - ■ have had recent nose ulcers or nose ...
-
Directions
Read insert (inside package) on how to: ■ get a new bottle ready (primed) before first use - ■ prime bottle again if not used for more than 2 weeks - ■ use the spray - ■ clean the spray ...
-
Other information
■ do not use if sealed package is torn or opened - ■ keep package and insert. They contain important information. ■ store between 20°-25°C (68°-77°F)
-
Inactive ingredients
benzalkonium chloride, carboxymethylcellulose sodium, dextrose anhydrous, edetate disodium dihydrate, hydrochloric acid or sodium hydroxide (for pH adjustment), microcrystalline cellulose ...
-
Questions or comments?
1-888-838-2872
-
Package/Label Principal Display PanelNASAL Allergy 24 HOUR Carton Text - Original Prescription Strength - Compare to Nasacort® Allergy 24 HR active ingredient - NDC 50804-085-17 - Multi-Symptom ·Nasal Allergy Spray - NASAL - Allergy ...
-
INGREDIENTS AND APPEARANCEProduct Information