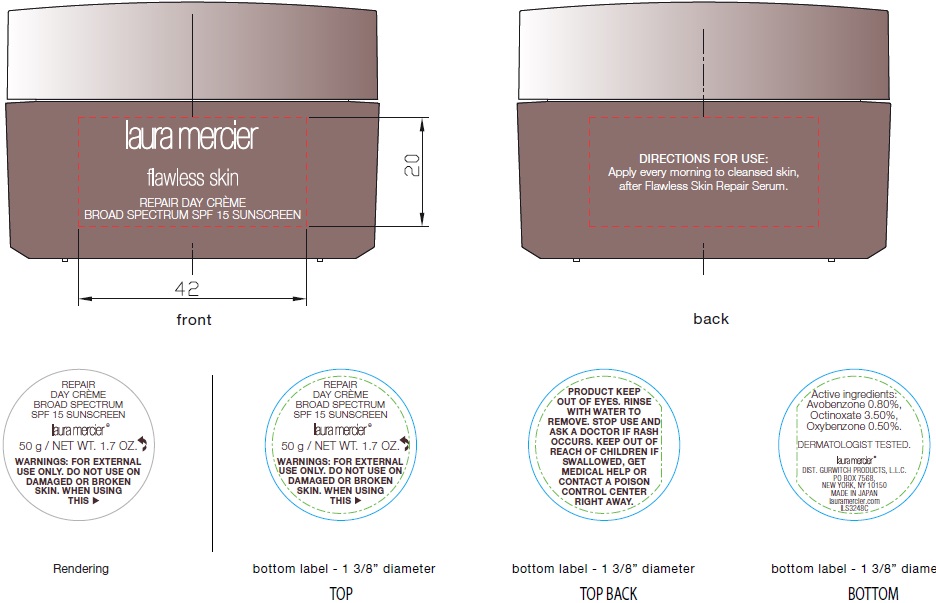
Label: LAURA MERCIER FLAWLESS SKIN REPAIR DAY BROAD SPECTRUM SPF 15 SUNSCREEN- avobenzone, octinoxate, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 65342-7500-1 - Packager: Gurwitch Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
• For sunscreen use:
• apply liberally 15 minutes before sun exposure
• use a water resistant sunscreen if swimming or sweating
• reapply at least every 2 hours
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• Children under 6 months of age: Ask a doctor - Other information
-
Inactive Ingredients
Water, Sea Water, Glycerin, Polymethylsilsesquioxane, Trehalose, Dimethicone, Squalane, Butylene Glycol, Polyglyceryl-10 Isostearate, Dicaprylyl Carbonate, Cetearyl Alcohol, Beeswax, Vitis Vinifera (Grape) Seed Oil, Hydrolyzed Xylan, Argania Spinosa Kernel Oil, Camellia Sinensis Leaf powder, Phenoxyethanol, Polyacrylamide, Carbomer, C13-14 Isoparaf n, Tocopheryl Acetate, PVM/MA Decadiene Crosspolymer, Disodium EDTA, Potassium Hydroxide, Pyrus Cydonia Seed Extract, Laureth-7, Glycosphingolipids, Glycerylamidoethyl Methacrylate/Stearyl Methacrylate Copolymer, Sodium Polyacrylate, Synthetic Fluorphlogopite, Aluminum Hydroxide, Hydrolyzed Collagen, Ethyl Oleate, Dipotassium Glycyrrhizate, Caprylic/Capric Triglyceride, Zinc Oxide, Tocopherol, Yeast Extract, Yeast Beta-Glucan, Sodium Ascorbyl Phosphate, Hydrogen Dimethicone, Oligopeptide-6, Tripeptide-3, Hexapeptide-3, Linalool.
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
LAURA MERCIER FLAWLESS SKIN REPAIR DAY BROAD SPECTRUM SPF 15 SUNSCREEN
avobenzone, octinoxate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65342-7500 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 8 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 35 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCERIN (UNII: PDC6A3C0OX) TREHALOSE (UNII: B8WCK70T7I) DIMETHICONE (UNII: 92RU3N3Y1O) SQUALANE (UNII: GW89575KF9) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) YELLOW WAX (UNII: 2ZA36H0S2V) GRAPE SEED OIL (UNII: 930MLC8XGG) ARGAN OIL (UNII: 4V59G5UW9X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) CYDONIA OBLONGA SEED (UNII: JXU526QH1V) LAURETH-7 (UNII: Z95S6G8201) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ZINC OXIDE (UNII: SOI2LOH54Z) TOCOPHEROL (UNII: R0ZB2556P8) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65342-7500-1 1 in 1 CARTON 01/05/2017 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/05/2017 Labeler - Gurwitch Products, LLC (884875246)