Label: prednisolone- Prednisolone solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0121-0687-05, 0121-0687-08, 0121-0687-16 - Packager: Pharmaceutical Associates, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 9, 2007
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
N/A - Section Title Not Found In DatabaseRx ONLY
-
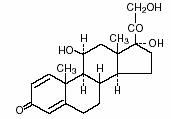
DESCRIPTIONPrednisoLONE Syrup (PrednisoLONE Oral Solution USP) contains prednisolone which is a glucocorticoid. Glucocorticoids are adreno-cortical steroids, both naturally occurring and synthetic, which are ...
-
CLINICAL PHARMACOLOGYNaturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic ...
-
INDICATIONS AND USAGEPrednisoLONE Syrup (PrednisoLONE Oral Solution USP) is indicated in the following conditions: Endocrine Disorders - Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone ...
-
CONTRAINDICATIONSSystemic fungal infections.
-
WARNINGSIn patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is ...
-
PRECAUTIONSGeneral - Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of ...
-
ADVERSE REACTIONSFluid and Electrolyte Disturbances - Sodium retention - Fluid retention - Congestive heart failure in susceptible patients - Potassium loss - Hypokalemic ...
-
DOSAGE AND ADMINISTRATIONDosage of PrednisoLONE Syrup (PrednisoLONE Oral Solution USP) should be individualized according to the severity of the disease and the response of the patient. For infants and children, the ...
-
HOW SUPPLIEDPrednisoLONE Syrup (PrednisoLONE Oral Solution USP) is a berry-flavored, red liquid containing 15 mg of prednisolone in each 5 mL (teaspoonful) and is supplied in 8 fl oz bottles (NDC 0121 ...
-
INGREDIENTS AND APPEARANCEProduct Information