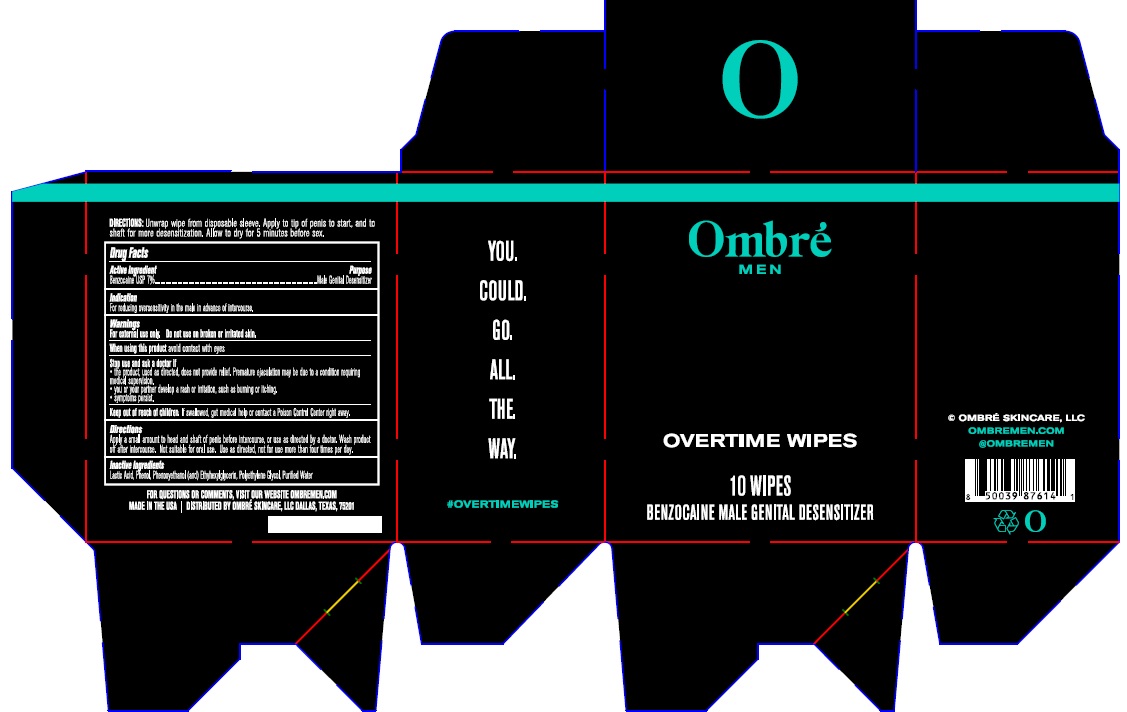
Label: OMBRE MEN OVERTIME WIPES- benzocaine cloth
- NDC Code(s): 54723-007-01
- Packager: Sambria Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Indication
-
Warnings
For External Use only. Do not use onbroken or irritated skin.
When using this avoid contact with eyes.
Stop and ask doctor if:- the product, used as directed, does not provide relief. Premature ejaculation may be due to a condition requiring medical supervision.
- you or your partner develop rash or irritation, such as burning or itching.
- symptoms persist.
- Keep out of reach of children
- Directions
- SPL UNCLASSIFIED SECTION
- Inactive ingredients
- Product label
-
INGREDIENTS AND APPEARANCE
OMBRE MEN OVERTIME WIPES
benzocaine clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54723-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 7 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) PHENOL (UNII: 339NCG44TV) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54723-007-01 10 in 1 PACKAGE 01/01/2022 1 3 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2022 Labeler - Sambria Pharmaceuticals, LLC (078676259)