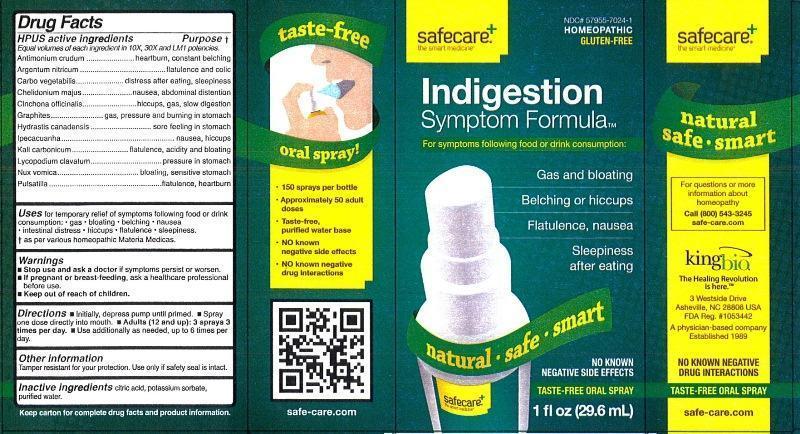
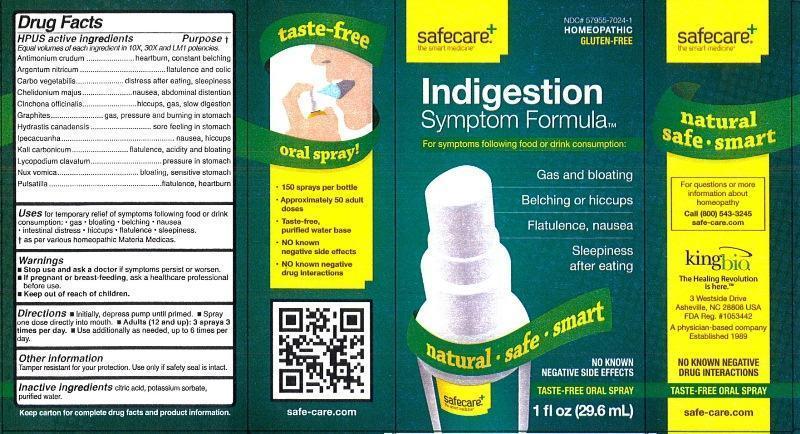
Label: INDIGESTION SYMPTOM FORMULA- antimonium crudum, argentum nitricum, carbo vegetabilis, chelidonium majus, cinchona officinalis, graphites, hydrastis canadensis, ipecacuanha, kali carbonicum, lycopodium clavatum, nux vomica, pulsatilla liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-7024-1 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 20, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- INACTIVE INGREDIENT
-
Purpose
HPUS active ingredients Purpose†
Equal volumes of each ingredient in 10X, 30X and LM1 potencies.
Antimonium crudum..................................................heartburn, constant belching
Argentum nitricum....................................................flatulence and colic
Carbo vegetabilis.....................................................distress after eating, sleepiness
Chelidonium majus...................................................nausea, abdominal distention
Cinchona officinalis...................................................hiccups, gas, slow digestion
Graphites.................................................................gas, pressure and burning in stomach
Hydrastis canadensis...............................................sore feeeling in stomach
Ipecacuanha............................................................nausea, hiccups
Kali carbonicum........................................................flatulence, acidity and bloating
Lycopodium clavatum...............................................pressure in stomach
Nux vomica...............................................................bloating, sensitive stomach
Pulsatilla...................................................................flatulence, heartburn
- Indication and Usage
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Dosage and Administration
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INDIGESTION SYMPTOM FORMULA
antimonium crudum, argentum nitricum, carbo vegetabilis, chelidonium majus, cinchona officinalis, graphites, hydrastis canadensis, ipecacuanha, kali carbonicum, lycopodium clavatum, nux vomica, pulsatilla liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-7024 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 10 [hp_X] in 29.6 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 29.6 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 10 [hp_X] in 29.6 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 10 [hp_X] in 29.6 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 10 [hp_X] in 29.6 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] in 29.6 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 10 [hp_X] in 29.6 mL IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 10 [hp_X] in 29.6 mL POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 10 [hp_X] in 29.6 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 10 [hp_X] in 29.6 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 10 [hp_X] in 29.6 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 10 [hp_X] in 29.6 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-7024-1 1 in 1 CARTON 1 29.6 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/15/2013 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-7024)