Label: METHYLENE BLUE injection, solution
- NDC Code(s): 14789-119-05, 14789-119-07
- Packager: Nexus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METHYLENE BLUE INJECTION safely and effectively. See full prescribing information for METHYLENE BLUE INJECTION. METHYLENE BLUE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS AND OPIODS
Methylene blue may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs and opioids. Avoid concomitant use of methylene blue with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs) and opioids. [see Warnings and Precautions ( 5.1) and Drug Interactions ( 7.1)].
Close -
1 INDICATIONS AND USAGEMethylene blue injection is indicated for the treatment of pediatric and adult patients with acquired methemoglobinemia.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage and Administration - Ensure patent venous access prior to administration of methylene blue injection. Do not administer methylene blue injection subcutaneously. Administer methylene ...
-
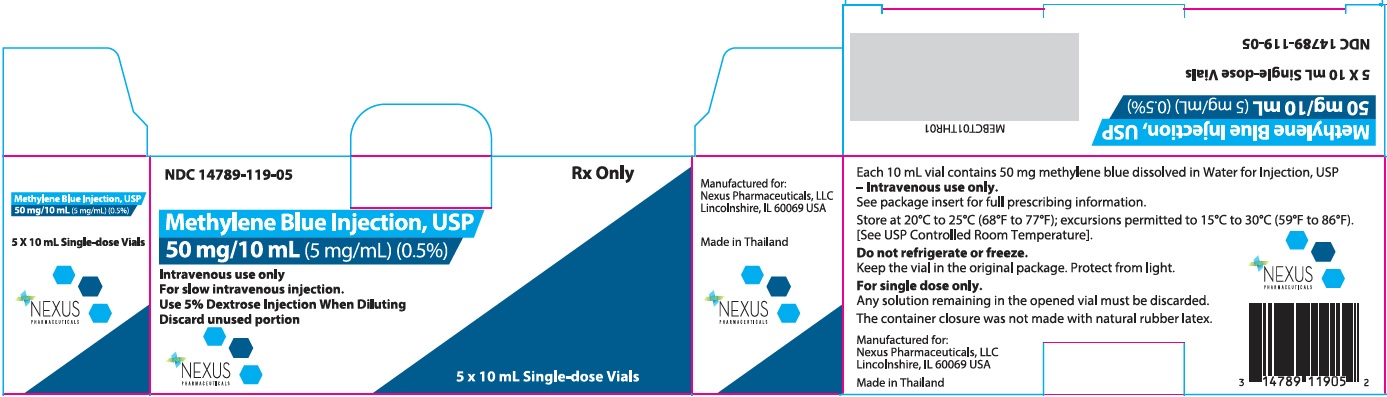
3 DOSAGE FORMS AND STRENGTHSMethylene blue injection:50 mg/10 mL (5 mg/mL) (0.5%) clear dark blue solution in single-dose vials.
-
4 CONTRAINDICATIONSMethylene blue is contraindicated in the following conditions: Severe hypersensitivity reactions to methylene blue or any other thiazine dye - [see Warnings and Precautions ( 5.2)] ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs and Opioids - The development of serotonin syndrome has been reported with the use of methylene blue class products. Most reports ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Serotonin Syndrome with Concomitant Use of Serotonergic Drugs - [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSClinically significant drug interactions with methylene blue are described below: The concomitant use of methylene blue with other drugs that affect the serotonergic neurotransmitter system has ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Methylene blue may cause fetal harm when administered to a pregnant woman. Intra-amniotic injection of pregnant women with a methylene blue class product during ...
-
10 OVERDOSAGEHypotension, wheezing and reduced oxygenation have been reported in patients who received methylene blue class products in single doses of 3 mg/kg or more. Administration of large intravenous ...
-
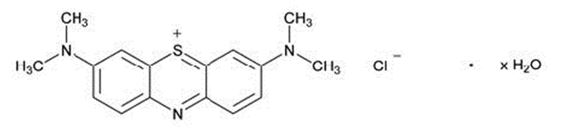
11 DESCRIPTIONMethylene blue is an oxidation-reduction agent. Its chemical name is 3,7-bis(dimethylamino)phenothiazin-5-ium, chloride hydrate. The molecular formula of methylene blue is C - 16H - 18ClN - 3S.xH ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Methylene blue is a water soluble thiazine dye that promotes a non-enzymatic redox conversion of metHb to hemoglobin. In situ, methylene blue is first converted to ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a two-year carcinogenicity study, rats were administered oral doses of methylene blue at 5, 25, or 50 mg/kg. Methylene blue caused ...
-
14 CLINICAL STUDIES14.1 Treatment of Acquired Methemoglobinemia - The efficacy of methylene blue in the treatment of patients with methemoglobinemia was evaluated in 24 adult patients with acquired ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMethylene Blue Injection, USP:is supplied in 10 mL single-dose vials. Each 10 mL vial contains 50 mg of methylene blue as a clear dark blue solution. A box contains five vials. Box of 5 single ...
-
17 PATIENT COUNSELING INFORMATIONSerotonin Syndrome - Advise patients of the possibility of serotonin syndrome, especially with concomitant use of serotonergic agents such as medications to treat depression and migraines ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 10 mL Carton Label - NDC 14789-119-05 - Rx Only - Methylene Blue Injection, USP - 50 mg/10 mL(5 mg/mL) (0.5%) Intravenous use only - For slow intravenous ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 10 mL Vial Label - NDC 14789-119-07 - Methylene Blue Injection, USP - 50 mg/10 mL(5 mg/mL) (0.5%) Intravenous use only - Use 5% Dextrose Injection When ...
-
INGREDIENTS AND APPEARANCEProduct Information