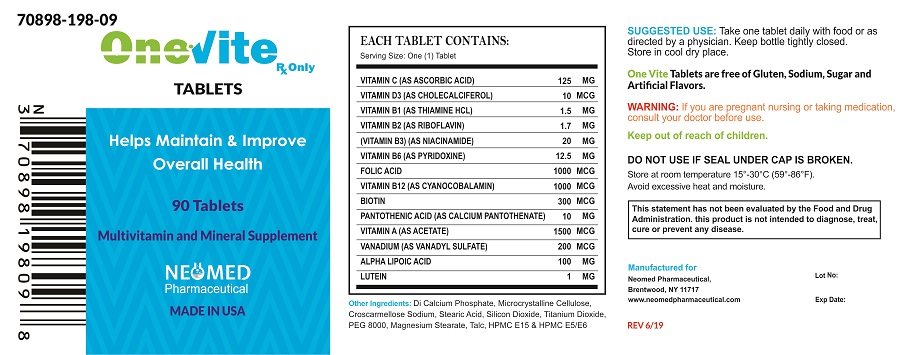
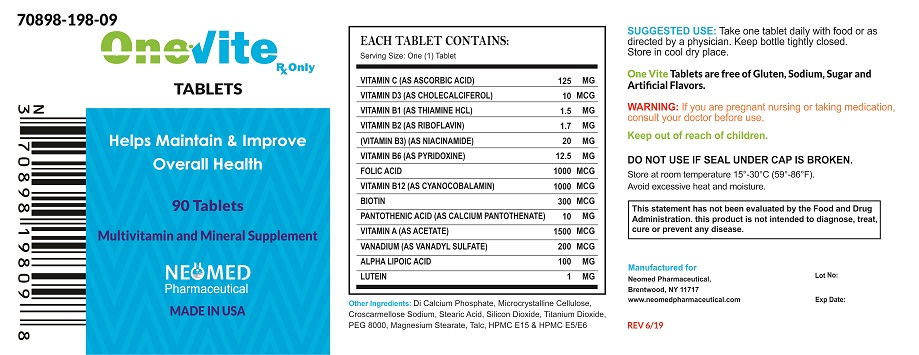
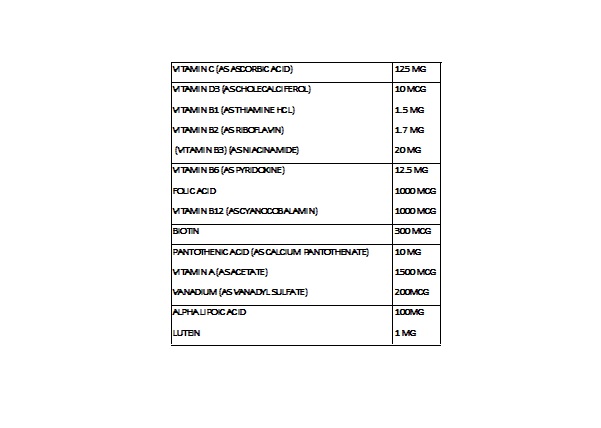
INDICATIONS AND USAGE
-
ONEvite Tablets is an orally administered prescription vitamin supplement specifically formulated for the clinical dietary management of patients with unique nutritional ...
INDICATIONS AND USAGE
ONEvite Tablets is an orally administered prescription vitamin supplement specifically formulated for the clinical dietary management of patients with unique nutritional needs requiring, increased folate levels and nutritional supplementation in physiologically stressful conditions. As well as for maintenance of good health and beneficial in maintaining overall skin health, also help maintain healthy blood sugar levels and help promote nerve health and function including normal vision.
CONTRAINDICATIONS
ONEvite Tablets is contraindicated in patients with hypersensitivity to any of its components. Folic Acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (Vitamin B12).
WARNING/PRECAUTIONS
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive.
The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Avoid Over dosage. Keep out of the reach of children.
Drug Interactions
High doses of folic acid may result in decreased serum levels of anticonvulsant drugs.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Consult appropriate references for additional specific vitamin-drug interactions.
Information for Patients
Patients should be counseled to disclose all medical conditions, including use of all medications, vitamins and supplements, pregnancy, and breastfeeding.
Pediatric Use
Not recommended for pediatric use.
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at levels substantially higher than those in ONEvite Tablets
DOSAGE AND ADMINISTRATION
ONEvite Tablets is a white film coating with caplet shape.
One tablet daily or as directed by a physician.
Close