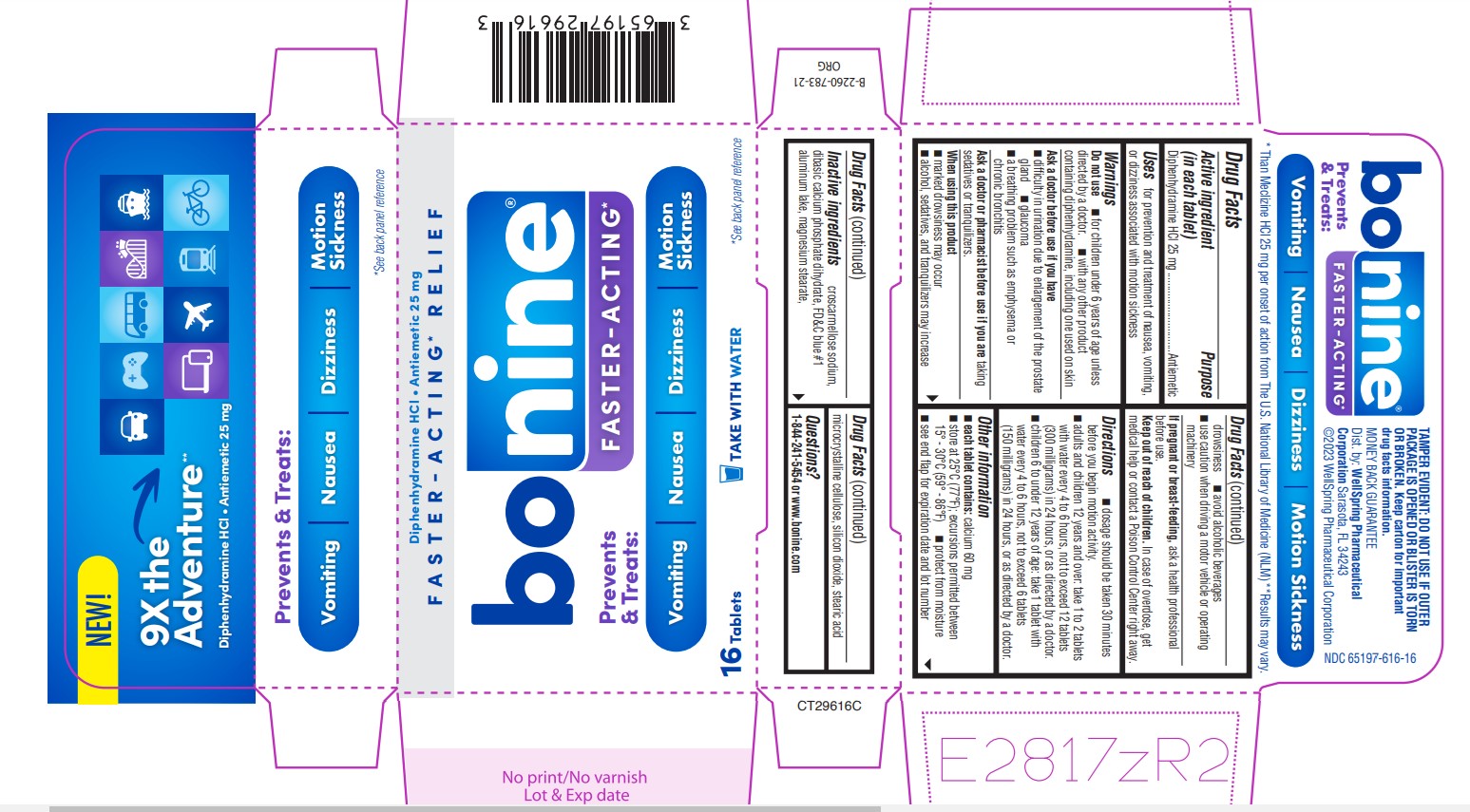
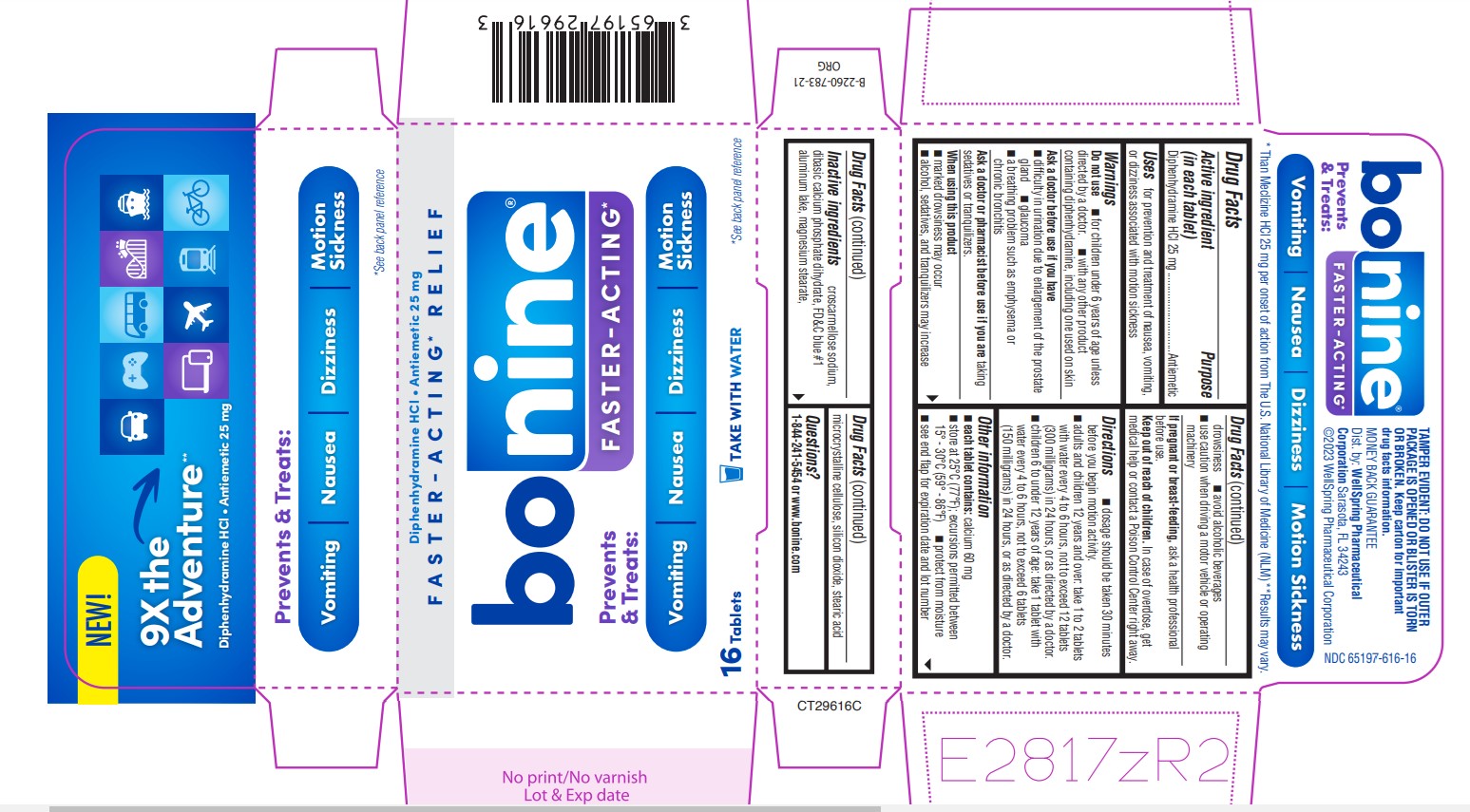
Label: BONINE FASTER-ACTING- diphenhydramine hcl tablet
- NDC Code(s): 65197-616-16
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
■ for children under 6 years of age unless
■ Do not use with any other product containing diphenhydramine, including one used on skin
Ask a doctor before use if you have
■ difficulty in urination due to enlargement of the prostate gland
■ glaucoma
■ difficulty in urination due to enlargement of the prostate gland
-
Directions
■ dosage should be taken 30 minutes before you begin motion activity.
■ adults and children 12 years and over: take 1 to 2 tablets with Water every 4 to 6 hours, not to exceed 12 tablets (300 milligrams) in 24 hours, or as directed by a doctor.
■ Children 6 to under 12 years of age: take 1 tablet with Water every 4 to 6 hours, not to exceed 6 tablets (150 milligrams) in 24 hours, or as directed by a doctor.
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL 65197-616-16
NEW
9X the Adventure**
Diphenhydramine HCl - Antiemetic 25 mg
Prevents & Treats:
Vomiting • Nausea • Dizziness • Motion Sickness
*See back panel reference
* Than Meclizine HCL 25 mg per onset of action from The U.S. National Library of Medicine (NLM)
** Results may vary
FASTER-ACTING* RELIEF
TAKE WITH WATER
Bonine Faster Acting 2.0
-
INGREDIENTS AND APPEARANCE
BONINE FASTER-ACTING
diphenhydramine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-616 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color blue Score no score Shape ROUND Size 9mm Flavor Imprint Code WS1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-616-16 2 in 1 BOX 05/01/2023 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part336 05/01/2023 Labeler - WellSpring Pharmaceutical Corporation (110999054)